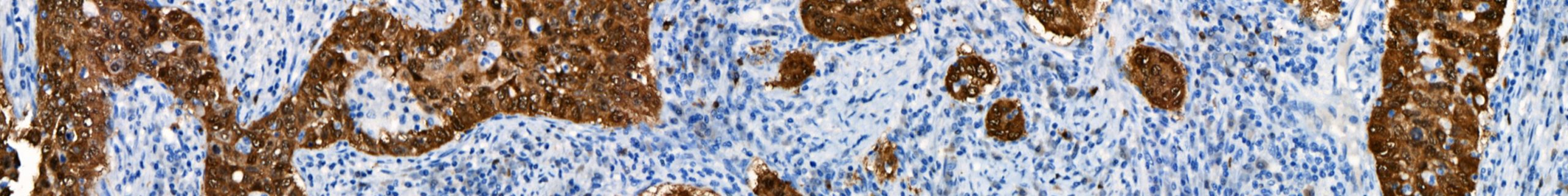
New p16 INK4a [BC42] monoclonal antibody with exceptional sensitivity and specificity San Francisco, CA, September 3, 2019 – Biocare Medical, LLC, a leading provider of innovative, automated immunohistochemistry (IHC) instrumentation and reagents, announces the release of a new mouse monoclonal antibody, p16 INK4a, for in vitro diagnostic (IVD) use in the qualitative identification of the […]
Approximately 12,000 men and women in the United States are diagnosed with gallbladder cancer annually, leading to an annual estimated 4,000 deaths.1 Gallbladder and other biliary cancers are comparatively rare in the United States, with about 0.7% of new cancer cases classified as gallbladder, contributing approximately 0.6% of cancer deaths yearly.1 Worldwide, gallbladder and other […]
Beyond the HIER 30-Slide Barrier The adoption of heat induced epitope retrieval, or HIER, as a standard enhancement technique for IHC has framed the use of IHC as a valuable tool for the examination of formalin fixed, paraffin embedded (FFPE) tissue for the past few decades.1 The mechanical requirements of heat induced epitope retrieval protocols […]
The VALENT, the Market’s Only Open, Fully Automated IVD Platform that Delivers Industry-Leading Throughput and Quality to Clinical, Pharmaceutical and Research Customers San Francisco, CA, January 24, 2019 – Biocare Medical, LLC, a leading provider of innovative, automated immunohistochemistry (IHC) instrumentation and reagents, announces the release of the VALENT IVD (in vitro diagnostic) automated staining […]
Biocare Medical is excited to announce that our new e-commerce platform is now online! We’ve made some significant changes to our online experience that we hope it will not only improve how you interact with us but save you time as well. Pay by Credit or Debit Card anytime Save Your Payment Method for Future […]
Excellere Partners has made an investment in Pacheco, California-based Biocare Medical, a provider of immunohistochemistry instrumentation, as well as a full range of reagents for IHC and molecular testing. No financial terms were disclosed. PRESS RELEASE Denver, CO, August 25, 2017 – Excellere Partners, a Denver-based private equity firm focused on partnering with entrepreneurs and management teams, […]
Biocare Medical, LLC is pleased to announce the availability of a comprehensive line of HPV probes for in situ hybridization. Each digoxigenin-labeled individual probe is designed to detect HPV DNA viral subtypes 6, 11, 16, 18, 31 or 51. Almost 80 million Americans are infected with Human Papilloma Virus (HPV), with about 14 million becoming […]
Concord, CA, August 27, 2015 – Biocare Medical is proud to announce the availability of CK HMW + p63 + AMACR (RM) as an IVD double-stain for Multiplex IHC™. This patented antibody cocktail may be useful in the evaluation of normal prostate glands, prostatic intraepithelial neoplasia (PIN) and prostatic adenocarcinoma (1,2). Previously, this product was […]
Biocare Medical, LLC is pleased to announce that the acquisition of New Windsor, NY based CymoGen Dx, LLC has been finalized. “We are very excited to be adding the portfolio of FISH [Fluorescent in situ Hybridization] probes from CymoGen Dx to our ever-expanding offerings in the Molecular Diagnostic arena,” said Roy Paxton Yih, President & […]
Biocare Medical, LLC has been awarded United States Patent No. US 8,852,592 for the anti-PAX8 mouse monoclonal antibody, clone [BC12]. This mouse monoclonal PAX8 antibody [BC12] has been designed to target a restricted epitope and exhibits higher specificity and provides sharper staining than the PAX8 rabbit polyclonal antibody. This clone was developed in-house and is […]