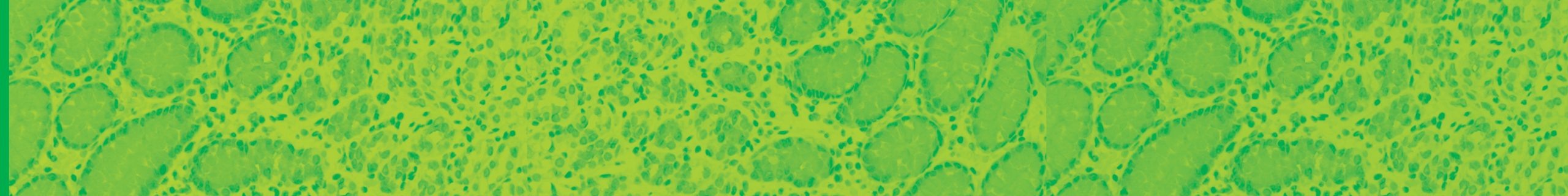
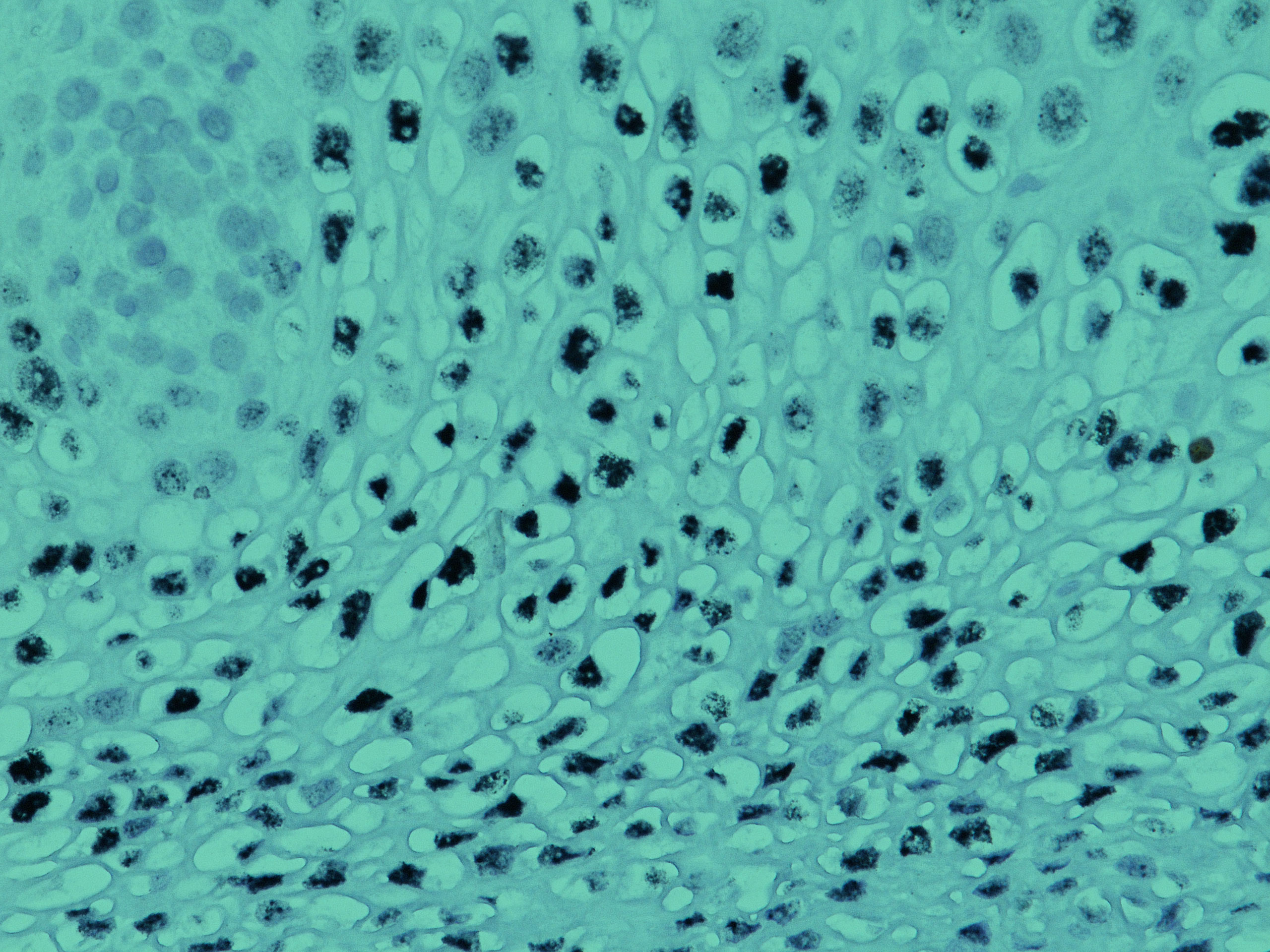
Claudins and Cancer: Promising Proteins for Detection and Diagnosis Claudin family proteins are transmembrane proteins that serve as major cell adhesion molecules of tight junctions. “Tight junctions restrict the flow of ions and aqueous molecules between cells, and their permeability is determined by the profile of claudin expression and arrangement of claudins with other proteins […]
Approximately 12,000 men and women in the United States are diagnosed with gallbladder cancer annually, leading to an annual estimated 4,000 deaths.1 Gallbladder and other biliary cancers are comparatively rare in the United States, with about 0.7% of new cancer cases classified as gallbladder, contributing approximately 0.6% of cancer deaths yearly.1 Worldwide, gallbladder and other […]
Approximately 13,000 women in the United States are diagnosed with cervical cancer annually, leading to an annual estimated 4,000 deaths.1 Cervical cancers are comparatively rare in the United States, with about 0.8% of new cancer cases classified as cervical, contributing approximately 0.7% of cancer deaths yearly.1 Worldwide, cervical cancer is the fourth most frequently diagnosed […]
Biocare Medical takes great pride in our promise of “Fighting Cancer, One Slide at a Time.” Our focus in the fight against cancer is realized through our high-quality immunohistochemistry (IHC) and in situ hybridization (ISH) reagents and instrumentation. Specifically, our sensitive and specific antibodies, molecular probes, and detection chemistries support early and accurate classification of […]