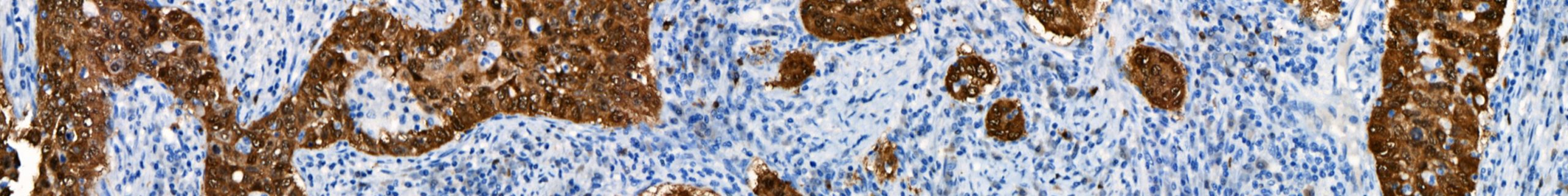
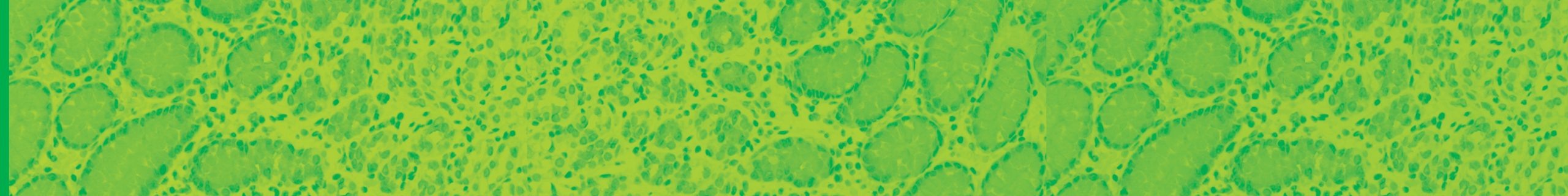
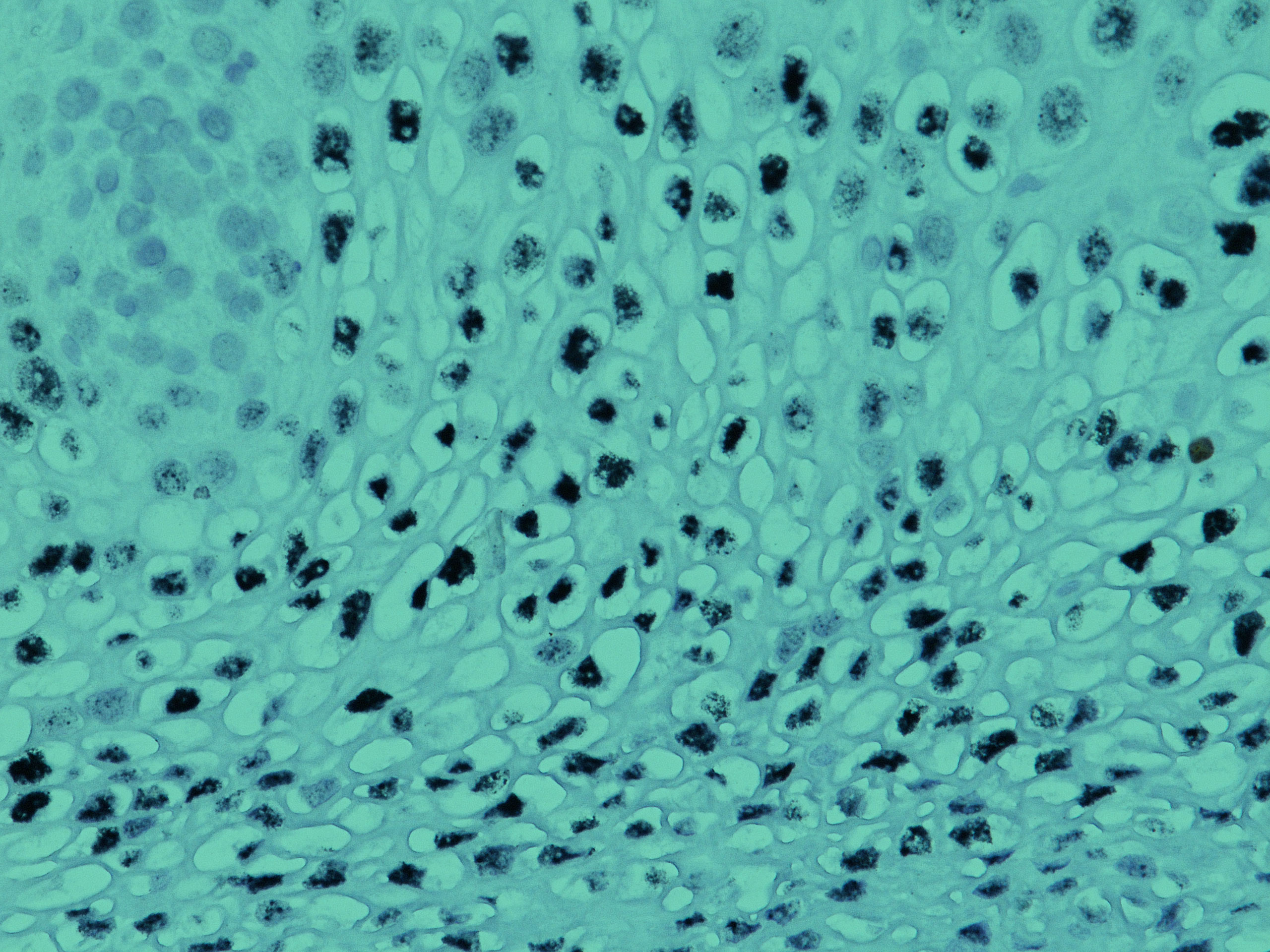
February 13, 2020 – Biocare Medical, a leading provider of innovative, automated immunohistochemistry (IHC) reagents and instrumentation, announces the launch of seven novel IVD IHC antibody markers for clinical diagnostics and research applications.The most recent launch focuses on several immuno-oncology markers, critical in aiding early-stage cancer drug developments and patient treatment. Biocare re-developed a major […]
New p16 INK4a [BC42] monoclonal antibody with exceptional sensitivity and specificity San Francisco, CA, September 3, 2019 – Biocare Medical, LLC, a leading provider of innovative, automated immunohistochemistry (IHC) instrumentation and reagents, announces the release of a new mouse monoclonal antibody, p16 INK4a, for in vitro diagnostic (IVD) use in the qualitative identification of the […]
Approximately 12,000 men and women in the United States are diagnosed with gallbladder cancer annually, leading to an annual estimated 4,000 deaths.1 Gallbladder and other biliary cancers are comparatively rare in the United States, with about 0.7% of new cancer cases classified as gallbladder, contributing approximately 0.6% of cancer deaths yearly.1 Worldwide, gallbladder and other […]
The VALENT, the Market’s Only Open, Fully Automated IVD Platform that Delivers Industry-Leading Throughput and Quality to Clinical, Pharmaceutical and Research Customers San Francisco, CA, January 24, 2019 – Biocare Medical, LLC, a leading provider of innovative, automated immunohistochemistry (IHC) instrumentation and reagents, announces the release of the VALENT IVD (in vitro diagnostic) automated staining […]
Approximately 13,000 women in the United States are diagnosed with cervical cancer annually, leading to an annual estimated 4,000 deaths.1 Cervical cancers are comparatively rare in the United States, with about 0.8% of new cancer cases classified as cervical, contributing approximately 0.7% of cancer deaths yearly.1 Worldwide, cervical cancer is the fourth most frequently diagnosed […]
Formalin has been used since 1893 as the standard fixative for tissue processing in histopathology.1 Due to formalin’s superior preservation of morphological detail, criteria for pathological diagnoses have been established through the observation of formalin-fixed, paraffin-embedded (FFPE) tissue sections stained with hematoxylin and eosin. Although many other fixatives exist, none have supplanted formalin in general […]
Biocare Medical takes great pride in our promise of “Fighting Cancer, One Slide at a Time.” Our focus in the fight against cancer is realized through our high-quality immunohistochemistry (IHC) and in situ hybridization (ISH) reagents and instrumentation. Specifically, our sensitive and specific antibodies, molecular probes, and detection chemistries support early and accurate classification of […]
Biocare Medical is excited to announce that our new e-commerce platform is now online! We’ve made some significant changes to our online experience that we hope it will not only improve how you interact with us but save you time as well. Pay by Credit or Debit Card anytime Save Your Payment Method for Future […]
Biocare Medical, LLC is pleased to announce the availability of a comprehensive line of HPV probes for in situ hybridization. Each digoxigenin-labeled individual probe is designed to detect HPV DNA viral subtypes 6, 11, 16, 18, 31 or 51. Almost 80 million Americans are infected with Human Papilloma Virus (HPV), with about 14 million becoming […]