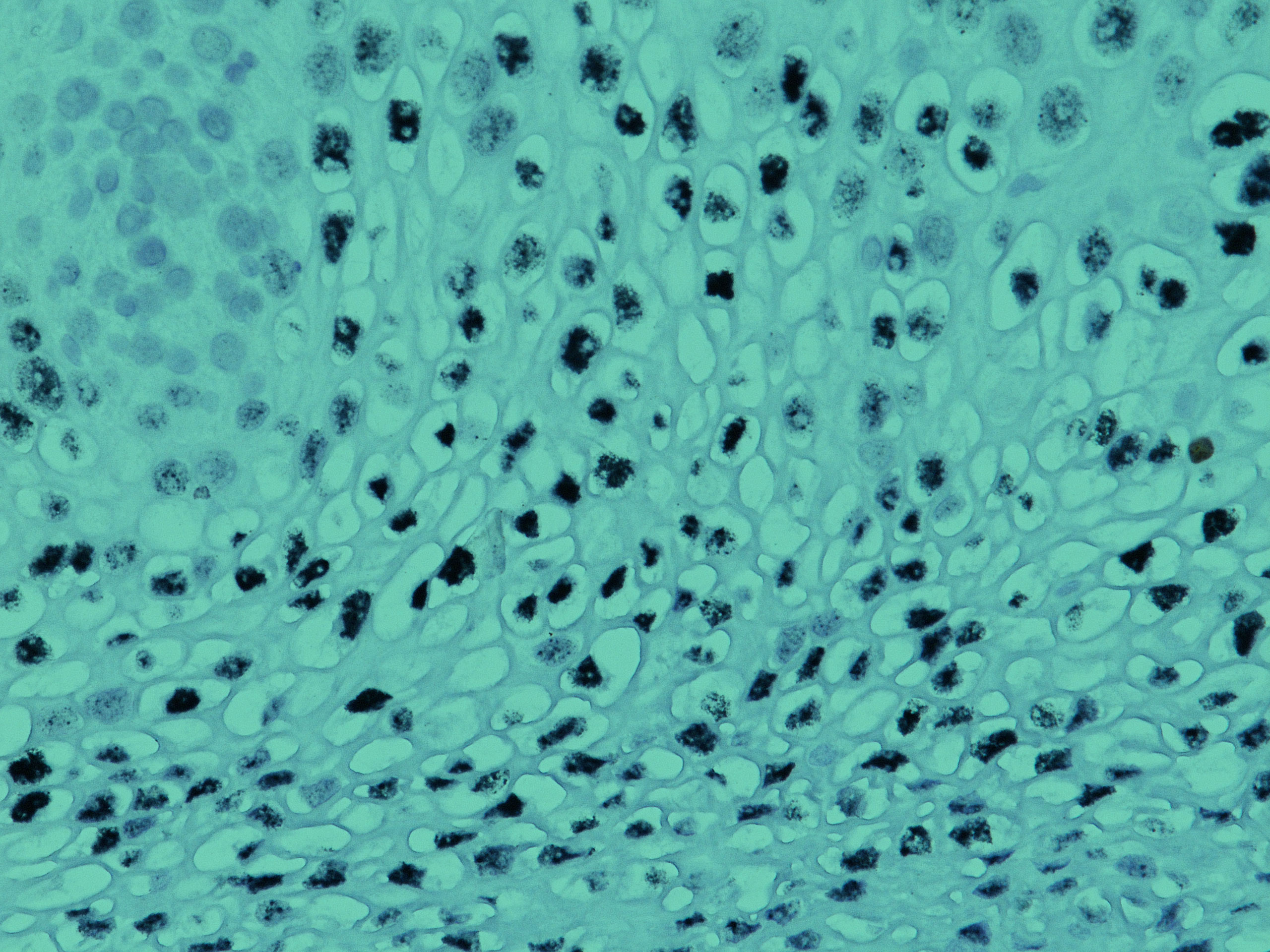
Beyond the HIER 30-Slide Barrier The adoption of heat induced epitope retrieval, or HIER, as a standard enhancement technique for IHC has framed the use of IHC as a valuable tool for the examination of formalin fixed, paraffin embedded (FFPE) tissue for the past few decades.1 The mechanical requirements of heat induced epitope retrieval protocols […]
The VALENT, the Market’s Only Open, Fully Automated IVD Platform that Delivers Industry-Leading Throughput and Quality to Clinical, Pharmaceutical and Research Customers San Francisco, CA, January 24, 2019 – Biocare Medical, LLC, a leading provider of innovative, automated immunohistochemistry (IHC) instrumentation and reagents, announces the release of the VALENT IVD (in vitro diagnostic) automated staining […]
Approximately 13,000 women in the United States are diagnosed with cervical cancer annually, leading to an annual estimated 4,000 deaths.1 Cervical cancers are comparatively rare in the United States, with about 0.8% of new cancer cases classified as cervical, contributing approximately 0.7% of cancer deaths yearly.1 Worldwide, cervical cancer is the fourth most frequently diagnosed […]
Formalin has been used since 1893 as the standard fixative for tissue processing in histopathology.1 Due to formalin’s superior preservation of morphological detail, criteria for pathological diagnoses have been established through the observation of formalin-fixed, paraffin-embedded (FFPE) tissue sections stained with hematoxylin and eosin. Although many other fixatives exist, none have supplanted formalin in general […]
Biocare Medical takes great pride in our promise of “Fighting Cancer, One Slide at a Time.” Our focus in the fight against cancer is realized through our high-quality immunohistochemistry (IHC) and in situ hybridization (ISH) reagents and instrumentation. Specifically, our sensitive and specific antibodies, molecular probes, and detection chemistries support early and accurate classification of […]
Utah Gastroenterology is the 2018 recipient of the Biocare Medical Excellence in Patient Care Award. The Utah Gastroenterology Histology Lab is a relatively new, small, private practice histology lab in Salt Lake City. The lab was nominated by the group of pathologists that provide diagnosis for the biopsies taken at Utah Gastroenterology. The pathologists were […]
Biocare Medical is excited to announce that our new e-commerce platform is now online! We’ve made some significant changes to our online experience that we hope it will not only improve how you interact with us but save you time as well. Pay by Credit or Debit Card anytime Save Your Payment Method for Future […]
Early on, Biocare recognized the market need for evaluating morphologically distinct markers to aid in solving complex clinical problems and simplifying interpretation, all while conserving precious patient tissue. The most glaring clinical need was determined to be the ability to differentiate between prostatic intraepithelial neoplasia (PIN) and carcinoma of the prostate. There also needed to […]
According to Fixation on Histology, Biocare Medical’s Dr. Jason Ramos, Ph.D. was awarded one of the top 5 best presentations at NSH 2018. For full details read below! “NSH’s Symposium/Convention is the largest histology conference in the United States with over 100 workshops, an exhibit hall, and plenty of opportunity to network with fellow histologists. […]
Immunohistochemical staining for programmed cell-death ligand 1 (PD-L1) in malignant thymoma and thymic carcinoma. Alexei Shimanovsky, Richard Cartun, Mary Fiel-Gan, Daniza Mandich, Jonathan Earle, Andrew L. Salner… Abstract e20003 Background: Recent development of anti-PD-1/L1 antibodies has demonstrated activity in various neoplasms. Thymic malignancies (TMS) are rare and treatment in advanced disease is limited. To evaluate […]