ERG
$220.00 – $778.00
Description
Product Description
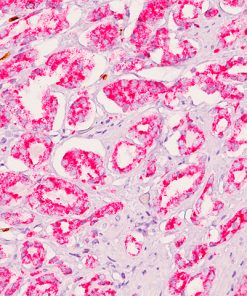
In human prostate cancer, the ERG oncogene is frequently overexpressed due to chromosomal translocations involving ERG and regulatory sequences of the TMPRSS2 or other androgen responsive genes. In particular, the TMPRSS2:ERG fusion gene has recently been found to be the most frequent gene rearrangement in prostate cancers, occurring in 45- 65% of North American patients. The mouse monoclonal anti-ERG antibody, clone 9FY, shows an unprecedented 99.9% specificity for detecting prostatic adenocarcinoma. Independent reports demonstrate 97-100% correlation between the expression of the ERG protein and the presence of TMPRSS2:ERG rearrangement and a remarkable concordance (96.5%) of ERG positive prostatic intraepithelial neoplasia (PIN) and ERG positive carcinoma in prostatectomy specimens.
Therefore, as a hallmark of the TMPRSS2:ERG chromosomal translocation, detection of ERG expression by 9FY offers a rare, but definitive marker of adenocarcinoma of prostatic origin, and unique opportunities to indicate oncogenic activations in PIN, to stratify prostate cancer patients for ERG oncogene status and to monitor treatment efficacy. Towards the stratification of patients, comparative evaluations of ERG protein expression status with 9FY and TMPRSS2-ERG gene fusions in hormone-naïve and castration resistant prostate cancers have shown promise for defining a subgroup of cases with dispensed androgen signaling pathway. Given the ease of performing IHC vs. FISH, ERG protein expression in formalin-fixed paraffin-embedded (FFPE) tissues may be an extremely useful tool for the routine identification of the ERG gene rearrangement and
diagnosis of prostatic adenocarcinoma.
Further utility of the mouse monoclonal anti-ERG antibody, 9FY, has been shown in detecting endothelial malignancies, including Kaposi sarcoma. Reports have also demonstrated the superior performance of 9FY in chromatin immunoprecipitation (ChIP), immunofluorescence (IF) and immunoblot assays.
Note: Clone 9FY [U.S. Patent 8,765,916 and patents pending] was developed by the Center for Prostate Disease Research with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Rockville, Maryland, USA.
Specifications
Specifications
| Intended Use | |
|---|---|
| Species Reactivity | |
| Source | |
| Clone | |
| Isotype | |
| Antigen | |
| Localization | |
| Positive Control |
Datasheets & SDS
| Download Data Sheet |
| Download RUO Data Sheet |
| Download SDS Sheet |
Regulatory Notice: Biocare’s IVD-labeled products comply with US-FDA and European IVDD regulation. Other regions may have additional requirements for such labeling, please contact your local distributor.
References
1. Petrovics G, Liu A, Shaheduzzaman S, Furasato B, Sun C, Chen Y, Nau M, Ravindranath L, Chen Y-D, Dobi A, Srikantan V, Sesterhenn IA, McLeod DG, Vahey M, Moul WJ, Srivastava S.: Frequent overexpression of ETS related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24, 3847-3852 (2005).
2. Rosen P, Sesterhenn IA, Brassell S, McLeod DG, Srivastava S, Dobi A.: Clinical potential of the ERG oncoprotein in prostate cancer. Nature Reviews Urology, 2012 Feb 14. doi: 10.1038/nrurol.2012.10.
3. Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA, Thangapazham R, Chen Y, McMaster G, Sreenath T, Petrovics G, McLeod DG, Srivastava S, Sesterhenn IA.: ERG oncoprotein expression in prostate cancer: clonal progression of ERG positive tumor cells and potential for ERG based stratification. Prostate Cancer and Prostatic Diseases 13, 228-237 (2010).
4. Braun M, Goltz D, Shaikhibrahim Z, Vogel W, Scheble V, Böhm D, Sotlar K, Fend F, Tan S-H, Dobi A, Wernert N, Perner S.: ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer – a comparative study of two monoclonal antibodies. Prostate Cancer and Prostatic Diseases, 2012 Jan 10. doi: 10.1038/pcan.2011.67.
5. Miettinen M, Wang Z-F, Paetau A, Tan S-H, Dobi A, Srivastava S, Sesterhenn IA.:ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. American Journal of Surgical Pathology 35, 432-441 (2011).
6. Mohamed AA, Tan S-H, Mikhalkevich N, Ponniah S, Vasioukhin V, Bieberich CJ, Sesterhenn IA, Dobi A, Srivastava S, Sreenath LT.: Ets Family Protein, Erg Expression in Developing and Adult Mouse Tissues by a Highly Specific Monoclonal Antibody. Journal of Cancer 1, 197-208 (2010).
7. Mohamed AA, Tan S-H, Sun C, Shaheduzzaman S, Hu Y, Petrovics G, Chen Y, Sesterhenn IA, Li H, Sreenath T, McLeod DG, Dobi A, Srivastava S.: ERG oncogene modulates prostaglandin signaling in prostate cancer cells, Cancer Biology and Therapy 11, 410-417 (2011).
8. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
9. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory workers from occupationally Acquired Infections; Approved guideline-Third Edition CLSI document M29-A3 Wayne, PA (2005).
Reviews (0)
Be the first to review “ERG” Cancel reply
You must be logged in to post a review.
This site uses Akismet to reduce spam. Learn how your comment data is processed.
Related products
B



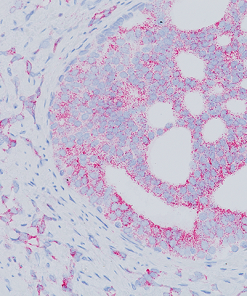
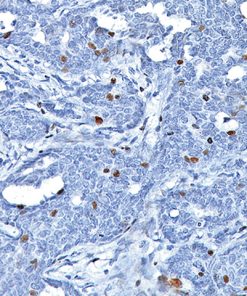
![(L) Anaplastic large cell lymphoma and (R) Lung adenocarcinoma stained with ALK antibody [5A4]](https://biocare.net/wp-content/uploads/3041-247x296.jpg)
Reviews
There are no reviews yet.