CD103 (RM)
$220.00 – $576.00
Description
Product Description
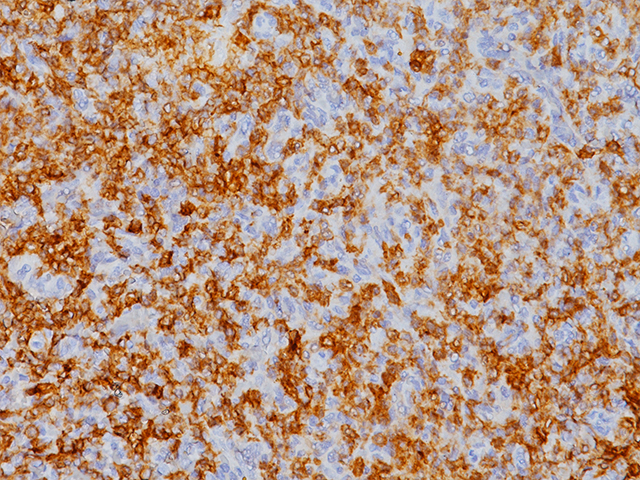
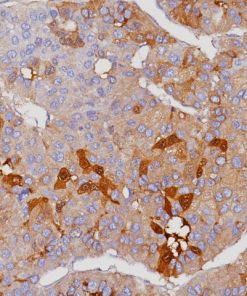
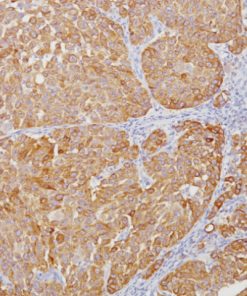
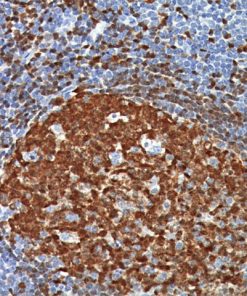
CD103 antibody recognizes the integrin subunit CD103 cell surface antigen, which is characteristically expressed in hairy cell leukemia (HCL), a B-cell lymphoproliferative disorder (1-3). CD103 [EP206] has demonstrated reactivity in FFPE (formalin-fixed paraffin-embedded) tissue, eliminating the need for flow cytometric analysis or frozen section IHC, making it a valuable addition to an immunohistochemistry (IHC) panel for the diagnosis of HCL (1). Other antibodies that have been used in conjunction with CD103 for the detection of HCL include CD25, TIA-1, DBA44 and CD11c (2-3). Intraepithelial CD8 (+) tumor-infiltrating lymphocytes (TIL) that express CD103 have been shown to be strongly associated with patient survival in high-grade serous ovarian cancer (HGSC) (4).
Specifications
Specifications
| Intended Use | |
|---|---|
| Format | |
| Volume | |
| Source | |
| Clone | |
| Isotype | |
| Antigen | A synthetic peptide corresponding to the residues of human CD103 protein |
| Localization | |
| Positive Control |
Datasheets & SDS
References
1. Morgan EA, et al. Immunohistochemical detection of hairy cell leukemia in paraffin sections using a highly effective CD103 rabbit monoclonal antibody. Am J Clin Pathol. 2013 Feb; 139(2):220-30.
2. Dong HY, et al. Immunophenotypic analysis of CD103+ B-lymphoproliferative disorders: hairy cell leukemia and its mimics. Am J Clin Pathol. 2009 Apr; 131(4):586 -95.
3. Mori N, et al. TIA-1 expression in hairy cell leukemia. Mod Pathol. 2004 Jul; 17 (7):840-6.
4. Webb JR, et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014 Jan 15; 20(2):434-44.
5. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
6. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014.
Related products
Primary Antibodies
B


![(L) Anaplastic large cell lymphoma and (R) Lung adenocarcinoma stained with ALK antibody [5A4]](https://biocare.net/wp-content/uploads/3041-247x296.jpg)