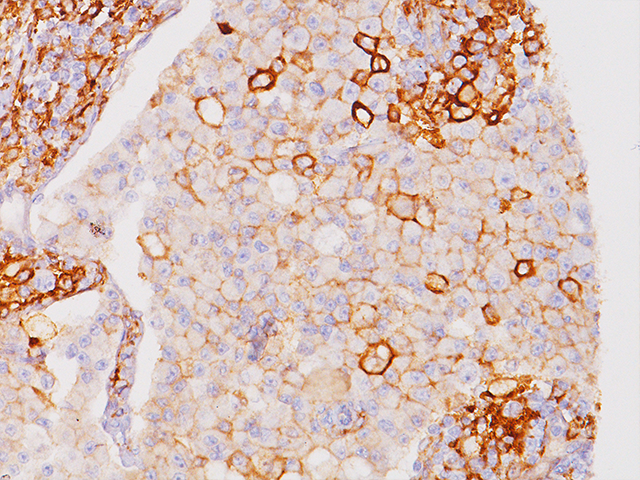
Several publications have compared Biocare’s PD-L1 (CAL10) antibody for immunohistochemistry to FDA approved PD-L1 clones SP263 and 28-8 in both rare and prevalent cancer types. Positive and significant correlation of expression, intensity and concordance resulted in both studies. Below is a recent abstract published in the Journal of Clinical Oncology using these different PD-L1 clones in malignant Thymoma and Thymic Carcinoma. Also included is a link to a study published in Human Pathology using the different PD-L1 clones in Breast Cancer.
Immunohistochemical staining for programmed cell-death ligand 1 (PD-L1) in malignant thymoma and thymic carcinoma. Alexei Shimanovsky, Richard Cartun, Mary Fiel-Gan, Daniza Mandich, Jonathan Earle, Andrew L. Salner… Abstract e20003
Background: Recent development of anti-PD-1/L1 antibodies has demonstrated activity in various neoplasms. Thymic malignancies (TMS) are rare and treatment in advanced disease is limited. To evaluate the potential impact of anti-PD-1/L1 therapy in TMS, we examined the expression of PD-L1 in previously resected thymoma (TM) and thymic carcinoma (TC). Methods: We examined resected specimens from patients at Hartford Hospital with TM and TC between 2000 and 2014. Expression of PD-L1 was evaluated on formalin-fixed paraffin-embedded tissue. Immunohistochemical testing was done using four different clones of PD-L1 antibodies on the Leica Bond Max automated platform. The four clones include: E1L3N (Cell Signaling Technology), 28-8 (Epitomics) and SP142 (Spring Bioscience), and CAL10 (BioCare). PD-L1 expression was evaluated based on the percentage of tumor cells positive and their intensity graded as negative, weak (1+), moderate (2+), and strong (+3). The scoring was performed by three pathologists and was blinded for clinicopathologic data and antibody clones. Results: We evaluated a total of 29 patients, including 26 patients with TM and 3 with TC. Among the 29 available specimens, 12 had completed PD-L1 expression assessment at the time of submission. PD-L1 expression is present in 75-100% of the evaluated patients. All had positive PD-L1 staining by SP142 and CAL10. Three patients showed strong intensity by CAL10, and one by SP142. E1L3N and 28-8 had positive PD-L1 expression in 9 and 8 patients respectively with weak/moderate intensity. SP142 and CAL10 demonstrated the strongest concordance (R2 = 0.91) but there was significant variation between antibodies (R2 = 0.31-0.91). No correlation was detected between tumor grade and PD-L1 expression. There were focal areas that lacked expression in all of the evaluated specimens. Conclusions: There is increased expression of PD-L1 in TMS. The level of PD-L1 expression varies between the four PD-L1 antibodies. Increased PD-L1 expression provides evidence for the use of PD-L1 inhibitors in TMS. The variable staining highlights the heterogeneity of TMS and challenges in developing predictive biomarker in this cancer.
Citations: Immunohistochemical staining for programmed cell-death ligand 1 (PD-L1) in malignant thymoma and thymic carcinoma. Alexei Shimanovsky, Richard Cartun, Mary Fiel-Gan, Daniza Mandich, Jonathan Earle, Andrew L. Salner, Katrina Collins, Gregory Alan Otterson, and Benjamin F. Chu. Journal of Clinical Oncology 2017 35:15_suppl, e20003-e20003 https://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.e20003