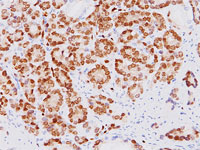
Biocare Medical is proud to announce the launch of the anti-ERG monoclonal antibody (clone 9FY CPDR) for in-vitro diagnostics, prognostics, patient monitoring, and screening by immunohistochemistry. The ERG oncoprotein is a promising diagnostic marker for identifying prostatic adenocarcinoma and distinguishing it from non-neoplastic prostate and other adenocarcinomas. This ERG antibody is 99.6% specific for prostate carcinoma and does not cross-react with infiltrating T- and B-Cells, unlike the alternative antibodies in the field.
Chromosomal translocations involving the ETS transcription factors, such as ERG and ETV1, are frequent events in human prostate cancer pathogenesis. In particular, the TMPRSS2-ERG fusion gene has recently been found to be the most frequent gene rearrangement in prostate cancers, occurring in 45-65% of North American patients. Recent reports have demonstrated a strong correlation between the presence of the TMPRSS2-ERG rearrangement and expression of the ERG protein, with ERG positive PIN associated with 90% of index tumors. Other studies have shown a strong concordance of prostatic intraepithelial neoplasm (PIN) positive ERG in 97% of patients. Therefore, as a hallmark of the TMPRSS2-ERG chromosomal translocation, ERG expression offers a rare, but definitive marker of adenocarcinoma of prostatic origin, and a unique opportunity to identify a potentially clinically significant subset of prostate cancer patients by IHC. Detection of HGPIN may indicate the need for subsequent biopsy.
Given the ease of performing IHC vs.. FISH, ERG protein expression in formalin fixed paraffin embedded tissue may be an extremely useful tool for the routine identification of the ERG gene rearrangement and diagnosis of prostate adenocarcinoma.
“I am highly impressed with the remarkable specificity and robustness of the ERG MAb in indentifying tumors cells in the prostate” says Dr. Sesterhenn, Chair of the Genitourinary Pathology at the AFIP.
About Biocare Medical
Biocare Medical LLC is an innovator in developing and supplying world class automated immunohistochemistry instrumentation, and the full range of reagents for IHC lab testing. Biocare is the market leader in simultaneous Multiplex IHC and antibody development which solves difficult clinical problems and accelerates turnaround time. The company’s customers include clinical histology laboratories, pharmaceutical companies, CROs, and biotechnology companies, as well as academic, government, military, and other non-profit laboratories. Biocare Medical offers an expanding portfolio of integrated products to address the rapidly growing cancer and infectious disease diagnostic and research markets using tissue immunostaining and in situ hybridization methods. Biocare Medical is headquartered and has manufacturing facilities in Concord, Calif., and has a global distribution network.
About CPDR
The Center for Prostate Disease Research is an internationally recognized inter-disciplinary program of the Department of Surgery of the Uniformed Services University of the Health Sciences (USU), the Department of Defense’s federal health sciences university in affiliation with the Walter Reed Army Medical Center (WRAMC), Armed Forces Institute of Pathology (AFIP), and many tri-service military medical centers, and is a collaboration with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.