Thinking of Automating Your IHC Lab?
Are you thinking about automating your IHC Lab? Do you want to increase daily throughput and volume of your lab? Do you need to standardize your staining procedures to reduce variability and improve staining quality? Do you need to reduce hands-on time, decrease cost per slide and better utilize lab personnel in value-added tasks?
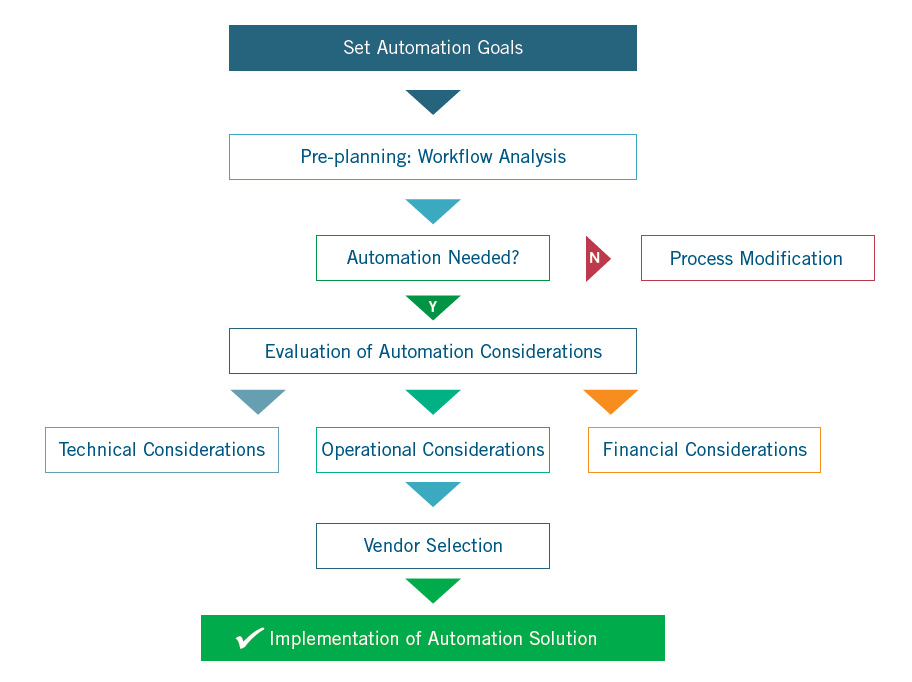
Continue reading and download the full white paper below. This detailed outline will walk you through all of the key considerations to be made before automating your lab such as reviewing your objectives, planning, technical, operational and financial considerations.
Common Objectives of Automating Your IHC Lab
- Increase daily throughput and volume of samples per technologist/technician.
- Standardization to reduce variability and to improve staining quality and consistency.
- Enhance cost efficency – minimize hands-on time and better utilize lab personnel in value-added tasks.
- Reduced errors through auto-correction, process monitoring and information tracking.
- Improve TAT as a result of fewer repeats and standardization.
Why IHC Automation?
The demand for IHC tests has been increasing since the 1990s as a result of advances in personalized medicine. In conjunction with a standard H&E stain, IHC data provides valuable information for many pathologists in the evaluation of neoplastic diseases. Based on market research data in 2007, it was estimated that about 20% of the slides examined by pathologists were IHC slides; additionally, data as recent as 2016 suggests that this share of volume of IHC tests remained the same if not increased this decade.1,2 Yet, the pathology community faces many different challenges in their IHC practice:
- The shrinking histotechnologist/histotechnician workforce as a result of fewer training opportunities and retirement.3
- Increasing pressure to reduce TAT: a key quality performance indicator for many clinical labs.4
- The difficulty in standardization of IHC tests and quality control. IHC is a process subject to substantial variation between different labs with different practices. There is a pressing need for standardization and quality control.5
- Reduced reimbursement for clinical labs. Since the implementation of PAMA in 2014 clinical labs face greater financial pressure with an average 35% decrease in reimbursement for most tests from 2018 to 2023.6 Finding ways to minimize cost and reduce repeat testing will be of paramount importance to sustain the business of clinical labs now and in the years to come.
All these environmental factors could be driving automation of the IHC process, but does it mean this is applicable to your lab? This white paper introduces the process and key factors to review and consider before bringing automation to your lab.
“Based on customer feedback, a daily throughput of 25 slides is the threshold for most laboratories to consider IHC automation.”


 Still not sure on how or where to start your automation journey? We are here to help, contact us today to speak to one of our highly-trained staff to walk you through the process step-by-step.
Still not sure on how or where to start your automation journey? We are here to help, contact us today to speak to one of our highly-trained staff to walk you through the process step-by-step.