Description
Product Description
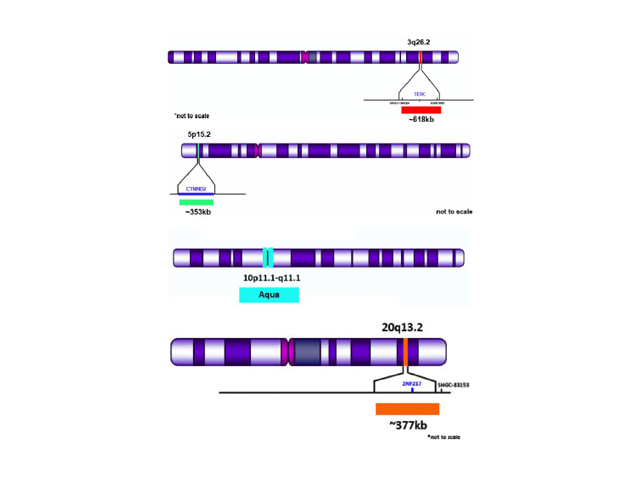
Cervical cancer is one of the most common cancers affecting women worldwide. The implementation of cytogenetic based screening techniques has greatly reduced the incidence and mortality of cervical cancer in the United States1. Moreover, traditional screening methods such as Pap smears and human papillomavirus (HPV) based testing are utilized to stratify and identify patients with high grade cervical cancer1. Furthermore, the utilization of fluorescence in situ hybridization (FISH) HPV-cervical cancer based tests allow for the detection of several hallmark cytogenetic abnormalities commonly identified in cervical carcinoma1, 2, 3. Specifically, chromosomal gains at TERC (3q26.2 ERC), 5p15.2, 20q13.2 and chromosomal losses at 10q are commonly associated with cervical cancer1, 2, 3. Conventional cytogenetic techniques such as FISH can be used to detect these chromosomal abnormalities.
Datasheets & SDS
| Download Data Sheet |
| SDS Sheet |
Browse more documents for this product (IFUs, datasheets, translations, SDS, and more).