IDO1
$237.00 – $974.00
Description
Product Description
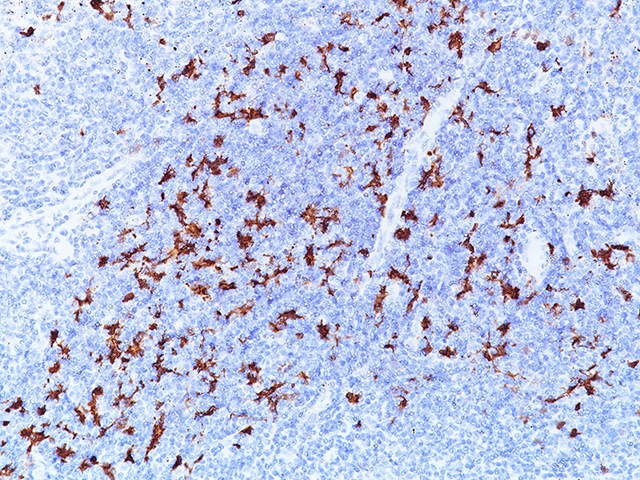
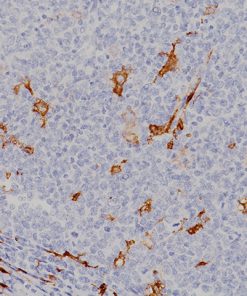
Indoleamine 2,3-dioxygenase 1 (IDO1) is a 403 amino acid cytoplasmic enzyme encoded by the INDO gene on human chromosome 8p22. IDO1 expression can be seen mainly in dendritic cells (DCs), such as immature and mature DCs, germinal center follicular DCs, interdigitating DCs, plasmacytoid DCs and in normal lymphoid organs, including lymph nodes, spleen, tonsils, Peyer’s patches, the gut lamina propria, and the thymus (1-2). In lymph nodes, tonsils, and Peyer’s patches, IDO1 staining was mainly observed in paracortical T-cell areas in cells that are larger than lymphocytes and tended to have an irregular outline, and often stellate with ramified cytoplasmic expansions. The spleen contains numerous IDO1-expressing cells in periarteriolar lymphocyte sheaths and some scattered positive cells in red pulp. The lamina propria of the duodenum, small, and large intestine contained interstitial mononuclear IDO1-positive cells, which were larger than lymphocytes. In the thymus, the expression of IDO1 was restricted to the medulla. Numerous interstitial cells, morphologically different from lymphocytes, were strongly positive, whereas a weak positivity was observed in rare epithelial cells, including Hassal’s bodies (2). In the bone marrow, a weak IDO1 expression is seen in very few cells, which could correspond to some stromal or DCs (2). In non-hematolymphoid tissue, IDO1 was detected in endothelial cells of many blood vessels in the lung, the prostate and the uterus (2). Many tumor types express IDO1 protein. However, the expression is often limited to a small number of tumor cells (2). Endometrial and cervical carcinomas most frequently expressed IDO1, followed by renal cell carcinomas, nonsmall cell lung carcinomas, and colorectal carcinomas (2). IDO1 expression in human tumors is usually restricted to three different cell types: myeloid cells, endothelial cells, and tumor cells in tumor and tumor-draining lymph nodes (2-3). IDO1 expression by malignant and nonmalignant cells can inhibit T-cell immune responses, leading to immune evasion and tumor outgrowth. Based on these findings, IDO1 inhibition using epacadostat, a selective small-molecule inhibitor of IDO1, was evaluated in a mouse model of cancer and in human solid tumors (3-4). It has been found that the inhibition of IDO1 can induce T-cell dependent antitumor immunity (3-4). This activity was related to the ability of epacadostat to block regulatory T-cell activity and promote dendritic cell maturation and function. Immunohistochemical detection of IDO1 expression on tumor-infiltrating immune cells and tumor cells should be very useful in evaluating IDO1 blockade and treatment efficacy (3-4).
Specifications
Specifications
| Format | |
|---|---|
| Volume | |
| Intended Use | |
| By Letter | |
| Antigen | |
| Clone | |
| Isotype | |
| Localization | |
| Source | |
| Positive Control |
References
1. Heitger A. Regulation of expression and function of IDO in human dendritic cells. Curr Med Chem. 2011;18:2222–33.
2. Theate I, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015; 3:161–72.
3. Beatty GL, et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin Cancer Res. 2017; 23:3269-76.
4. Kristeleit R, et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)–only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol (2017) http://dx.doi.org/10.1016/j.ygyno.2017.07.005.
5. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
6. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014.
Datasheets & SDS
| Download Data Sheet |
| Download SDS Sheet |
Browse more documents for this product (IFUs, datasheets, translations, SDS, and more).


![(L) Anaplastic large cell lymphoma and (R) Lung adenocarcinoma stained with ALK antibody [5A4]](https://biocare.net/wp-content/uploads/3041-247x296.jpg)