Differentiating Diluents: Tricks of the Trade for Diluting Antibodies
Aqueous environment greatly influences antibody conformations. When preparing working solutions of concentrated antibodies, it is important to select a diluent that will not alter the stability and binding properties of the antibody. Charge, hydrophobicity, and other physiological/chemical properties can affect antibody-diluent combinations.
Binding of antigens to antibodies is pH dependent and highly controlled by ionic interactions. Diluents with a relatively neutral pH (pH of 7.0-7.4) are often ideal buffers and a great starting point for optimization. “The isoelectric point, or pI (the pH at which the net electric charge of a molecule is zero), for immunoglobulins can range from 5.8 to 8.5 for a given antibody.”¹ If the pH of the diluent is too close to the pI of the antibody, solubility can weaken, resulting in negative effects on staining and background. Amino acid side chains in antibodies and antigens are also affected by pH. For example, at pH 6.0, histidine amino acids are mostly positively charged and attracted to negative charges on the antigen, but at a pH of 7.9, histidine amino acids are mostly uncharged and are repelled by negative charges on the antigen. Therefore, an antibody diluent should be chosen to be compatible with the predominating attractive forces for the particular antibody and antigen. Additionally, common diluent additives, such as salts and azides, used to “reduce non-specific interactions among charged molecules”¹, can affect ionic strength and alter antibody interaction. Excessive ionic strength can lead to decreased reactivity of antibody-antigen binding.
Below are a few important things to consider before diluting the primary antibody:
• Don’t assume all antibody diluents are the same
• Do test the concentrated antibody with different diluents (varying pH and composition) if one is not recommended by the antibody manufacturer.
• Don’t significantly alter the pH of the diluent buffer when optimizing
• Do raise and lower the pH by 0.5 pH units if low signal or high background is observed.¹
• Don’t use diluents that contain serum if not necessary; serum can bind to the primary antibody causing decreased sensitivity or even false positive results if binding to the secondary antibody
• Do use caution if a serum-free option is not available; perhaps try an endogenous protein block separate from the diluent.
• Don’t frequently or rapidly change the temperature of the diluent/working solution, as refrigeration and heating may change the pH of temperature sensitive buffers (especially Tris based buffers).¹
• Do allow diluent/working antibody solution to reach room temperature before using. Solutions should be prepared at the temperature in which they will be used.
Biocare Medical offers a full line of antibody diluents that are specially formulated to improve antibody titers and are extremely stable for long term antibody storage. In most cases, when compared against other Phosphate Buffered Saline (PBS) based and Tris-based diluents, antibody titers may be improved 2-4-fold. Greater primary antibody dilutions may provide cost-savings, higher specificity, and reduce nonspecific background staining. Biocare’s diluents are ready to use and protease and IgG free. For greater convenience, all Biocare Medical’s primary antibodies are tested with all Biocare’s diluents and the diluent producing optimal results is recommended on the data sheet.
To learn more about Biocare Medical’s antibody diluent offering, please call 800-799-9499 or visit our website:
https://biocare.net/products/ancillaries/antibody-diluents/
1. Immunohistochemical Staining Methods, Sixth ed. Dako Denmark A/S, An Agilent Technologies Company, 2013.
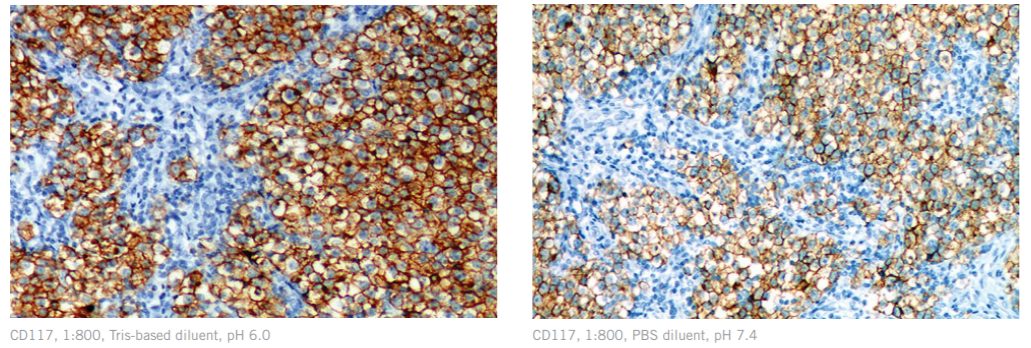
CD117 diluted in Biocare’s Renoir Red, 1:800 (L) vs. CD117 diluted in a standard PBS diluent, 1:800 (R)
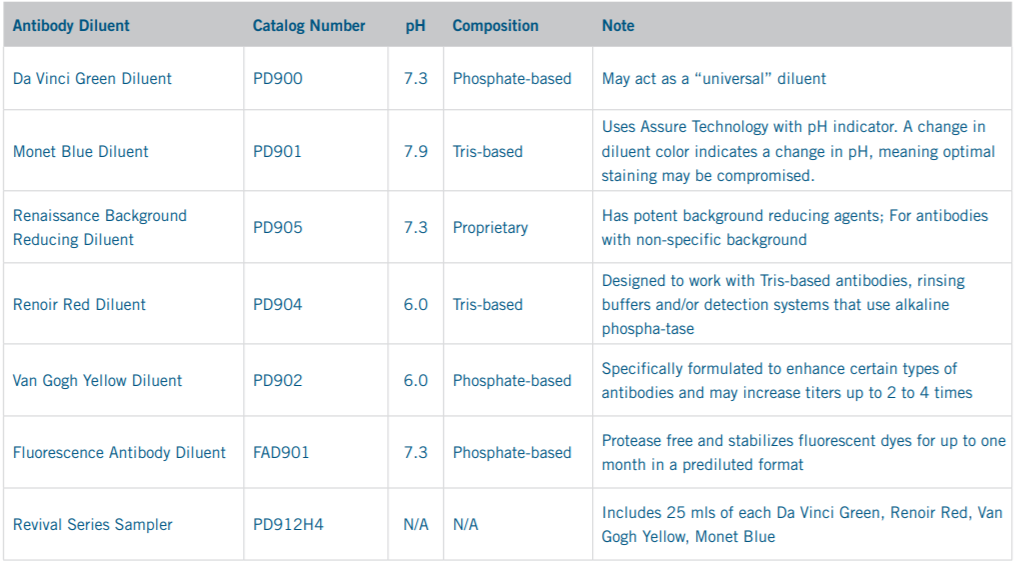
Biocare Medical’s Antibody Diluent Offering