Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group and Biocare Medical, LLC (Biocare) today announced that they have entered into a non-exclusive license agreement to allow Biocare access to certain patents and materials related to p63 diagnostics in the research and IVD field. In parallel, Biocare and AsymmetRx Medical, Inc. (AsymmetRx) have settled their dispute as it relates to the p63 technology. As part of the settlement, Biocare has gained a worldwide license through Ventana to distribute p63 (4A4) mouse monoclonal primary antibody in both the research and IVD market and AsymmetRx has agreed to terminate all patent infringement litigation. AsymmetRx holds the exclusive, worldwide license under the Harvard Medical School patent filings for the use of the p63 antibody as an aid in the diagnosis of prostate and other cancers. Financial terms of the license and settlement were not disclosed.
“Our mission is to improve the lives of all patients afflicted with cancer,” says Mara Aspinall , President, Ventana Medical Systems, Inc. “Through our licensing program, we make the most advanced products in the industry available to those in need and help ensure the standardization of diagnostic results to benefit patients.”
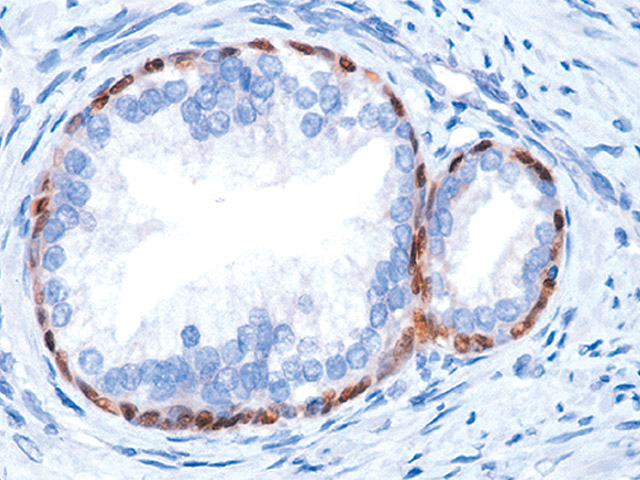
The p63 gene was discovered in the Harvard lab of Dr. Frank McKeon and the p63 cancer diagnostic test was invented by a collaboration of labs at Harvard Medical School, Dana Farber Cancer Institute and the Brigham and Women’s Hospital. p63 protein is highly expressed in prostate, breast, bladder, and lung tissue and the p63 (4A4) mouse monoclonal antibody has been extremely useful in the differentiation of benign and malignant lesions in conjunction with morphological findings. Numerous articles in top clinical journals have cited the accuracy and utility of p63 as an aid in prostate cancer diagnosis, and it has become the gold standard used by leading hospitals, laboratories and experts worldwide.
“Including p63 in our lung, prostate and breast panels really empowers our Multiplex IHC platform to aid pathologists in making critical decisions and solidifies our passion for patient care and the ability to fight cancer one slide at a time,” says Roy Paxton Yih , President & CEO, Biocare Medical, LLC.
“Prostate cancer is one of the most difficult cancers to diagnose, and the p63-based test adds a significant advantage to making the correct diagnosis. We are very excited about our agreement with Ventana, and now Biocare, as it will provide a platform to bring the p63 diagnostic tests to patients worldwide,” says Peter F. McKeon , President, AsymmetRx Medical, Inc.
p63 (4A4) mouse monoclonal primary antibody.
About Ventana Medical Systems, Inc.
Ventana Medical Systems, Inc. (“VMSI”) (SIX: RO, ROG; OTCQX: RHHBY), a member of the Roche Group, innovates and manufactures instruments and reagents that automate tissue processing and slide staining for cancer diagnostics. VENTANA solutions are used in clinical histology and drug development research laboratories worldwide. The company’s intuitive, integrated staining, workflow management platforms, and digital pathology solutions optimize laboratory efficiencies to reduce errors, support diagnosis and inform treatment decisions for anatomic pathology professionals. Together with Roche, VMSI is driving Personalized Healthcare through accelerated drug discovery and the development of “companion diagnostics” to identify the patients most likely to respond favorably to specific therapies.
VENTANA and the VENTANA logo are trademarks of Roche.
Visit www.ventana.com to learn more.
Ventana Medical Systems, Inc.
Empowering | Innovation
VMSI Media Relations
Jacqueline Bucher
Senior Director, Corporate Communications
Phone: 520-877-7288
e-mail: jacquie.bucher@ventana.roche.com
Request High Resolution Files
About Biocare Medical, LLC
Biocare Medical, LLC is an innovator in developing and supplying world class automated immunohistochemistry (IHC) instrumentation, and the full range of reagents for IHC lab testing. Biocare Medical is the market leader in simultaneous Multiplex IHC and antibody development, which aids in the assessment of clinical cases and accelerates turnaround time. The company’s customers include clinical histology laboratories, pharmaceutical companies, CROs, and biotechnology companies as well as academic, government, military, and other non-profit laboratories. Biocare Medical offers an expanding portfolio of integrated products to address the rapidly growing cancer and infectious disease diagnostic and research markets using tissue immunostaining and in situ hybridization methods. Biocare Medical is headquartered and has manufacturing facilities inConcord, California, and has a global distribution network. For more information, please visit www.biocare.net.
About AsymmetRx Medical
AsymmetRx Medical, Inc. is privately held and was founded in 2001 by scientists from Harvard University and Johns Hopkins University to develop novel diagnostics, imaging agents, and therapies for urologic cancers including those affecting the bladder and prostate. Using strategies based on key advisors from private and academic clinicians and pathologists specializing in urologic cancers, AsymmetRx is committed to developing the most accurate tests for the analysis of prostate and bladder biopsies, as well as early detection tests that in the future will obviate the need for needle biopsies. AsymmetRx is committed to developing systems of imaging urologic cancers that will aid in the early treatment of such diseases, as well as therapies based on specific targeting of malignant cells in urologic cancers. The Wall Street Journal recognized AsymmetRx for one of the “Best Technologies” in the Biotech medical field in 2005. AsymmetRx succeeded in developing the first FDA cleared test for prostate cancer in needle biopsies based on the p63 antibody. It is anticipated that the p63-based test will assist in the accurate diagnosis and treatment of hundreds of thousands of men in the United States alone. More detailed information on the company and its mission may be found at www.asymmetrxmedical.com.
© 2013 Ventana Medical Systems, Inc.
VENTANA and the VENTANA Logo are trademarks of Roche.
All other trademarks are the property of their respective owners.