Simplifying the Process of ‘Switching’ Antibodies and Verifying the Performance of a Modified IHC Procedure
Has your laboratory ever considered ‘switching’ from one vendor’s antibody to another to obtain improved results and/or reduce reagent costs, but wondered what doing so might mean in terms satisfying various regulatory-agency standards? If so, you probably know that all immunohistochemistry (IHC) procedures employed for clinicaldiagnostic purposes must be appropriately validated prior to placing them into service. However, a relatively new approach advocated by the College of American Pathologists (CAP) may simplify the verification of procedures in which use of the same antibody clone is an integral part of the ‘switch’.
The process of validating all laboratory procedures – including IHC – is outlined in U.S. Clinical Laboratory Improvement Amendments (CLIA) regulations1, and, for laboratories that are voluntarily accredited by the CAP, outlined in that organization’s IHC-specific standards. As most individuals who work in the IHC area of a diagnostic pathology lab will agree, the process of validating an IHC procedure is time-consuming, relatively expensive, and often requires that several pathologists – who may not agree on the microscopically-observed reactivity of a given IHC procedure – review and approve the applicable staining protocol.
Clearly, subjectivity to the interpretation of IHC assays can affect how well a modified procedure might be accepted.
Another reason that the validation process can be challenging is because it requires a significant amount of effort to identify positive-(and negative-) ‘control’ specimens that should be stained in order to
confirm that the assay is capable of identifying different biological patterns of expression for a given protein. In other words, finding enough of the ‘right’ positive/negative control tissues can be difficult, especially in labs that only receive a few types of specimens, such as in gastroenterological, dermatological, and urological environments. So, if ways can be identified in which to reduce the quantity of specimens/ slides that must be tested, doing so is a very worthwhile objective.
One of the most common and long-held beliefs is that initial IHC procedure validation must involve testing 10 to 20 ‘positive’ specimens, each with varying degrees of reactivity. The truth is that the quantity of positively reactive and negatively reactive specimens that are tested during the validation process is at the discretion of each lab’s medical director and must be described in the lab’s policy/ procedure manual. So, what about changing from one source (vendor) of a given antibody to another? Does this require a lab to “start from scratch” and determine the effectiveness of the modified procedure in the same manner as they would for an altogether new assay (antibody)? The answer to that question is “maybe” and depends on the nature of the procedural modification, as described in more detail below. To reduce any confusion that may exist with the associated terminology, the process of confirming the effects of modifying a previously validated IHC assay should be referred to as “verification” rather than “re-validation”.
Although a great deal of information is available in the published literature on the process of initial procedure validation2-4, until fairly recently, very little guidance was available on the steps that should be taken when only one or two parameters of a previously-validated procedure – such has heat-induced epitope retrieval (HIER) reagents/ methods or the antibody – are modified. Prior to the publication of procedure-verification standards5 by the CAP in 2014, each laboratory had to decide, and describe in policy form, the procedural changes that triggered the need to verify the performance of a modified IHC assay. Then, through its updated standards, the CAP began recommending that:
A) “When an existing validated assay has changed in any one of the following ways – antibody dilution, antibody vendor (same clone), or incubation or retrieval times (same method) – the laboratory should confirm assay performance with at least 2 known positive and 2 known negative cases; and
B) When any of the following have changed – fixative type; antigen retrieval method (e.g. change in pH, different buffer, different heat platform), antigen detection system, tissue processing or testing equipment, environmental conditions of testing (e.g. laboratory relocation), or laboratory water supply – laboratories must confirm assay performance by testing a sufficient number of cases to ensure that assays consistently achieve expected results”.
Based on these new guidelines, it should be clear that a laboratory is not required to “start from scratch” when verifying the performance of a modified procedure, especially when doing so involves the same antibody clone as was used in the previously validated procedure. And what if your lab is not accredited by the CAP? In this case, since CLIA regulations do not specifically address the verification of modified IHC procedures, it is simply a good practice to follow well-established guidelines such as those developed by the CAP5,6. So, what exactly does ‘post-modification’ procedure verification involve? The most important steps include:
1) Testing a small set of positive and negative specimens and comparing the staining results of the modified assay with the staining results of the existing assay – with the goal being that the modified assay is ‘as good as’ (if not better) than the existing assay;
2) Documenting the parameters that were modified and the outcome of the testing; and
3) Documenting the approval of the modified and verified assay by the lab’s medical director before it is placed into routine clinical use.
An example of the information that might be gathered during the procedure verification process is shown in sample form shown on
next page:
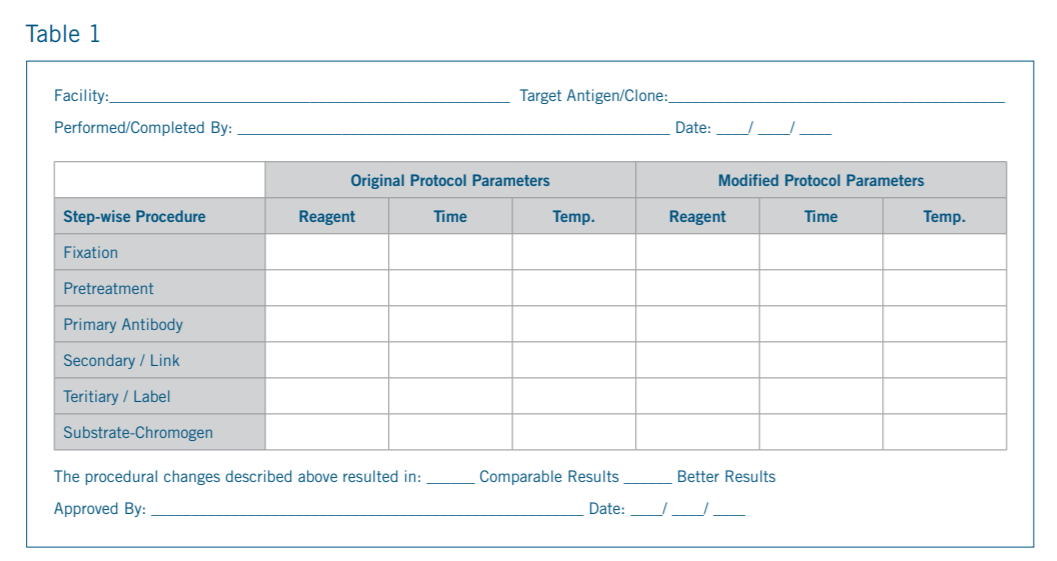
In conclusion, verifying the performance of a modified IHC procedure is not as difficult as once thought The keys are understanding the expected effects of the proposed procedural change before proceeding, carefully evaluating the actual effects during the testing and verification process, and ensuring the laboratory’s medical director approves the procedural change. For additional insight into the validation and verification of IHC procedures, please review the guidelines outlined in the publication that led to the new CAP standard6.
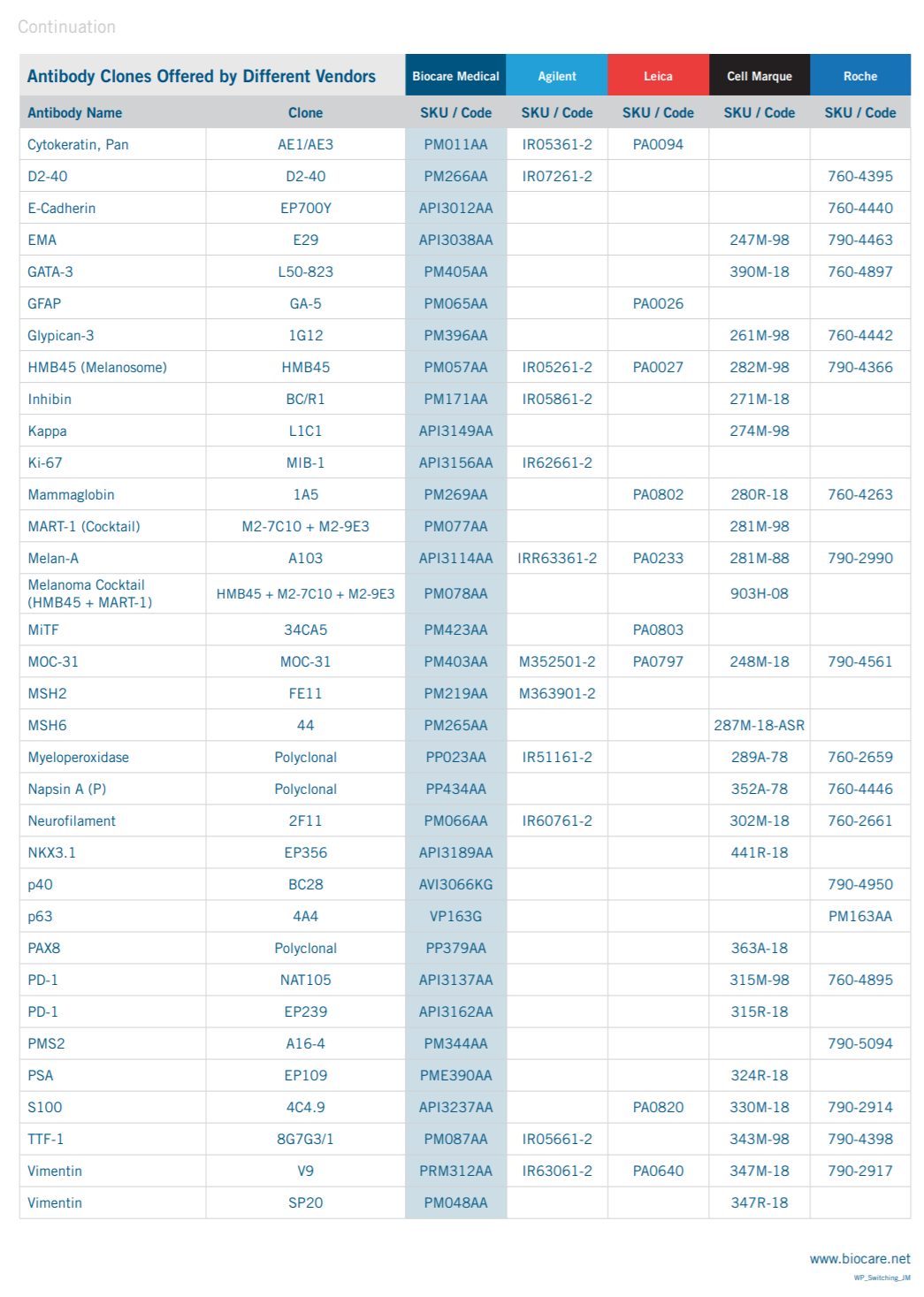
If your lab is considering modifying an existing IHC procedure to obtain improved staining results and/or reduce reagent costs, the table below outlines the similarities and differences between the antibody clones offered by Biocare Medical and several other leading antibody vendors. Please call Biocare Medical at 1-800-799-9499 or visit our website at www.biocare.net for additional information.
1. Clinical Laboratory Improvement Act (CLIA) Regulations: 42CFR493.1253 through 493.1281. (http://wwwn.cdc.gov/clia/regs/toc.aspx).
2. Taylor CR and Cote RJ, eds. Theoretical and Practical Aspects of Test Performance. In Immunohistology: A Diagnostic Tool for the Surgical Pathologist, 3rd. ed., Volume 19 In Major Problems in Pathology. Philadelphia, PA: W.B Saunders, 2005.
3. Goldstein, NS, et.al. Recommendations for improved standardization in immunohistochemistry. Appl Immunohistochem Molec Morph 2007;15:124-133.
4. Quality Assurance for Design, Control, and Implementation of Immunohistochemistry Assays: Approved Guideline, Second Edition; Vol. 31, No. 4, 2011. Clinical and Laboratory Standards Institute (CLSI), Wayne PA, USA; Publication code: I/LA28-A2.
5. Anatomic Pathology Checklist, 2014. Laboratory Accreditation Program. College of American Pathologists. Waukegan, IL.
6. Fitzgibbons, et.al. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 2014;138:1-12