p40 according to NordiQC – BC28 Clone Performs Optimally on Run 60
Throughout the year, NordiQC conducts testing of key immunohistochemistry (IHC) reagents to ensure the continued performance of certain clones and IHC approaches. The results are published based on run data collected from laboratories all over the world and can also be correlated with internal testing within the expertise of NordiQC histologists and scientists. Most recently, NordiQC completed Run 60, 2020 and published results related to many IHC antibodies. In particular, the evaluation of the technical performance and level of analytical sensitivity and specificity of the IHC assays for p40 was performed by the NordiQC participants for the differentiation between lung squamous cell carcinoma and lung adenocarcinoma.¹
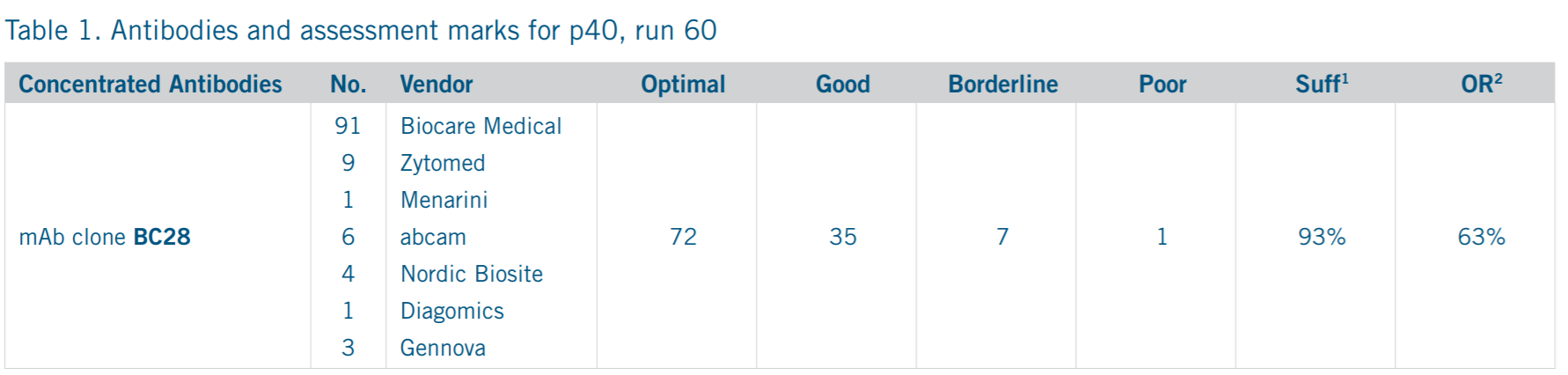
The testing of p40 (BC28) was conducted in 115 laboratories with 93% reporting sufficient marking and 107 technicians reporting optimalto-good staining results under the test conditions. The pass rate in this run improved from 74% (2016) to 86% (2020) and can be attributed to the use of ‘highly sensitive and robust mAb (monoclonal) clone BC28 both as concentrate and Ready-To-Use (RTU) format.’¹ In a note to the flexibility of BC28 across the IHC testing spectrum, NordiQC noted ‘the concentrated formats of mAb clone BC28 provided optimal staining results on the main platforms from Dako, Leica, and Ventana’.¹ NordiQC also discouraged the use of polyclonal p40 variants as those products ‘gave less successful results and should be avoided’.¹
Biocare remains committed to ensuring the high-quality reagents and performance noted by NordiQC, specifically related to p40 (BC28). Two products from Biocare were positively mentioned by NordiQC and can be found readily available for testing and implementation by labs running all types of IHC equipment. The standard offering of mAb clone BC28 can be found using catalog identifier PI3066, while a version specifically designed for use on the Ventana Ultra (Biocare Ultraline™offering) can be found using catalog identifier AVI3066KG.
IHC for p40 in two laboratories: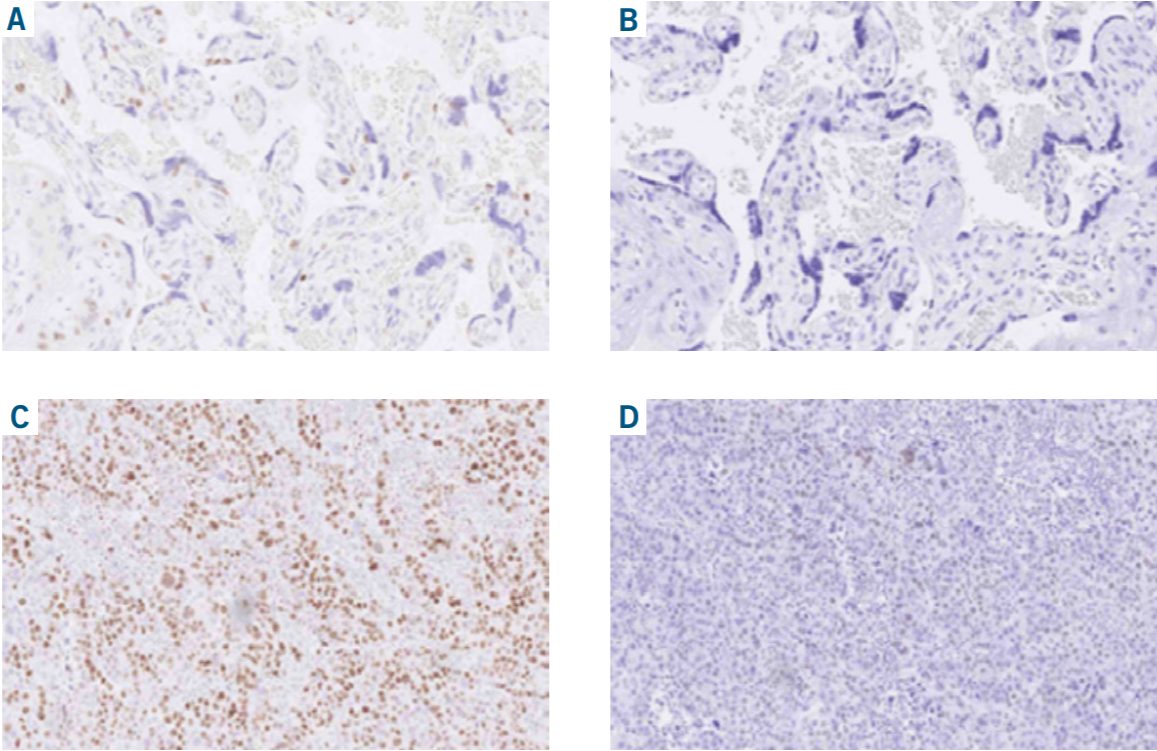
Lab 1 (A,C): Optimal results in placenta and lung squamous cell carcinomal using the mAb clone BC28 carefully calibrated to demonstrate p40 in tissues with both high- and low- level p40 expression.
Lab 2 (B,D): Insufficient result using a polyclonal Ab for p40 by protocol settings with too low level of analytical sensitivity and false negative results in both cytotrophoblasts in placenta and most neoplastic cells in the lung squamous cell carcinoma.
Interested in testing p40 (BC28) in your lab? Contact Biocare Medical to learn more about this antibody at 1-800-799-9499 or click the link here:
https://biocare.net/product/p40-antibody/
1. Assessment run 60 2020. https://nordiqc.org/downloads/assessments/136_10.pdf. December 14, 2020.
2. Results of Run 60. B30, H18, C8. Nodiqc.org/news.php#news_39. December 14, 2020.

