Automating Your IHC Lab Key Considerations
Why IHC Automation?
The demand for IHC tests has been increasing since the 1990s as a result of advances in personalized medicine. In conjunction with
a standard H&E stain, IHC data provides valuable information for many pathologists in the evaluation of neoplastic diseases. Based on
market research data in 2007, it was estimated that about 20% of the slides examined by pathologists were IHC slides; additionally,
data as recent as 2016 suggests that this share of volume of IHC tests remained the same if not increased this decade.
1,2 Yet, the pathology community faces many different challenges in their IHC practice:
- The shrinking histotechnologist/histotechnician workforce as a result of fewer training opportunities and retirement.
- Increasing pressure to reduce TAT: a key quality performance indicator for many clinical labs.
- The difficulty in standardization of IHC tests and quality control. IHC is a process subject to substantial variation between
different labs with different practices. There is a pressing need for standardization and quality control. - Reduced reimbursement for clinical labs. Since the implementation of PAMA in 2014 clinical labs face greater financial
pressure with an average 35% decrease in reimbursement for most tests from 2018 to 2023. Finding ways to minimize cost and reduce repeat testing will be of paramount importance to sustain the business of clinical labs now and in the years to come
All these environmental factors could be driving automation of the IHC process, but does it mean this is applicable to your lab? This
white paper introduces the process and key factors to review and consider before bringing automation to your lab.
Objectives of IHC Automation
- Increase daily throughput and volume of samples per technologist/technician.
- Standardization to reduce variability and to improve staining quality and consistency.
- Enhance cost effiency – minimize hands-on time and better utilize lab personnel in value-added tasks.
- Reduced errors through auto-correction, process monitoring and information tracking.
- Improve TAT as a result of fewer repeats and standardization
“Based on customer feedback, a daily throughput of 25 slides is the
threshold for most laboratories to consider IHC automation.”
Planning for IHC Automation, Workflow Analysis
Workflow analysis provides a clear, data-driven picture of what the lab is doing “right now.” The objective data delivers a comprehensive
understanding of the strengths and weaknesses of the specific lab workflow and can pinpoint with clarity the exact areas that the lab
needs to focus on to provide the biggest impact on efficiency. Workflow analysis also captures the attention of all stakeholders regarding
the introduction of a new automation solution.
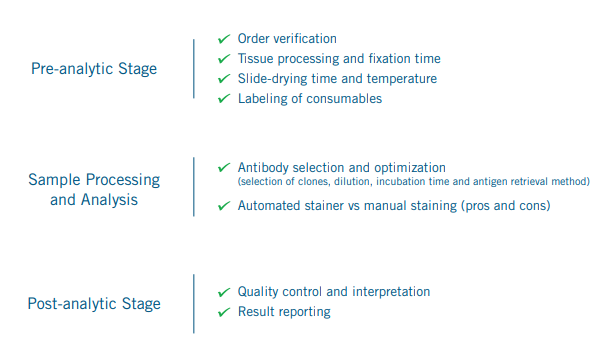
Workflow analysis needs to be done in the context of the entire sample life cycle as shown above.
7,8 Some of these are mundane tasks in pre-analytic/post-analytic stages (e.g. order verification, labeling of consumables, sample storage and data handling and reporting) but
perhaps the most important consideration here is in the automation of samples processing and analysis. Nonetheless, in some cases,
merely changing the current workflow may provide improved efficiency without the addition of automation. Steps in a typical workflow
analysis include:9,10,11
- Workflow mapping to identify functions and steps to be automated.
- Collect existing process performance data (e.g. TAT, cost) as baseline metrics.
- By means of quality improvement methods such as Lean Six Sigma
- Identify bottlenecks and roadblocks.
- Evaluate simple work modifications and process automation as solutions to eliminate bottlenecks and problems.
- Prioritize the potential modifications and automation solutions
After the conclusion of a workflow analysis, there should be no surprise that automation may not be the best solution for every lab.
Once the solutions to streamline current workflows and to automate processes have been implemented, the new automated process will
be monitored to ensure the intended objectives are attained and to make any necessary adjustments to the new process for maximum
performance and throughput.
Considerations for an Investment in IHC Automation
The topic has been reviewed in various literature with comparisons of the different automated IHC platforms in the market.
12,13 It is important to understand that there is no one best IHC system in the market. Rather, a solution applicable to your lab would be
an outcome of the interplay between the following factors and each lab will have its own unique challenges and requirements to be
considered.
Technical Considerations
There are three important technical factors that a lab manager needs to consider in automating the IHC process:
1. Degree of standardization of the IHC process.
2. How flexible the system would need to be? (Closed vs Open Platforms)
3. Is a fully-automated system the appropriate solution? (Full automation vs Semi-automation)
Standardization
Does the software enable better tracking of sample and order information to minimize errors and the need for repeats? Is connectivity
to LIS an important consideration for my lab?
Features such as reagent tracking, volume sensing, bulk liquid detection, and automated error detection/reporting could be important
in standardization and ensuring consistency of staining.
Reagent dispensing mechanism (matrix vs rotary platforms) is an important consideration.
Flexibility: Closed vs Open Platforms
Is it necessary for my lab to have a diverse choice of reagents (including antibody, detection) from any source?
Do I need the flexibility to modify different parameters in my protocol (e.g. incubation time, temperature, etc.)?
Do you require new biomarkers in the future? (If yes, an “open” system may offer more advantages)
Availability of RTU reagents so users can have the flexibility to switch to a “closed” system if required.
Is standardization and consistency of staining most important to my lab? How tolerant of error and variability are the current processes
in my lab? (Most pertinent for clinical applications where a large menu of RTU reagents may be required)
Do I need to have onboard heating for HIER? (This feature is usually not available in an “open” system)
Full Automation vs Semi-automation
What volume of slides is my lab handling daily? Weekly? Monthly? Do you expect that number to increase?
Do I need to include the entire IHC process from deparaffinization, to coverslipping on one platform? Does it make sense to have some
of the steps done separately? (e.g. antigen retrieval, coverslipping – utilize workflow analysis to determine)
Is there any operational constraint (e.g. TAT) that would make a fully automated platform a better choice?
Operational Considerations
Each lab has its own operational needs; the introduction of any new automation platform should evaluate the following:
Volume of Slides and TAT
Capacity of the system and volumes of samples: this is perhaps the most important parameter and will impact the slide
management functionalities required such as continuous processing and random slide processing. Slide capacity, batch size,
number of positions for reagent containers, multiplexing protocol and incubation time of different reagents will also have a
cumulative impact on the throughput of a platform. Most IHC automation platforms have a 30-50 slide capacity, some load
slides in trays of 5-10 slides while others are loaded one slide at a time. Every lab will need to find its sweet spot of balancing
system capacity, batch size and protocol mix in order to select the right option to achieve the best throughput. How does your lab
receive and process slides? All at one time? Do you receive a few slides at a time? Are they all the same test type?
Turnaround time (TAT): another key performance metric and important to clinician satisfaction which may trigger the need for
managing STAT requests in the automated IHC platform without impact on in-process slides. The definition of TAT is a matter
of debate and there are differences between clinicians and labs. It should be noted that TAT may be intra-laboratory versus total
TAT spanning from order placement to the point when the results are available for clinicians.
Management of Slides, Reagents and Waste
How many different protocols need to be implemented on the platform and how diverse are these protocols? Is multiplexing
required? Is it also necessary to run ISH tests on the platform?
Safety and Waste management: DAB is a hazardous waste requiring special handling and higher disposal cost. The capability to
separate DAB waste from other non-hazardous waste will make the disposal easier and more cost-effective as well as minimizing
histotechnologists’ exposure to hazardous materials.
Software and LIS Capability
How intuitive is the software (notably the user interface)? How easy is it to train the existing lab workforce? Is there a provision
(e.g. camera, barcode scanner) to recognize and track reagents and slides through the run?
Additional Considerations
Review technical support and training provided by vendors and how responsive they are; so that instrument downtime is minimized
and your automation solution has longevity as a lab workhorse.
Maintenance requirements for instrument include stringent cleaning as any slide staining protocol causes residue build-up which
significantly impacts staining quality. A provision of assisted-cleaning capabilities (i.e. part of the routine cleaning tasks can be
performed automatically) will be a big plus for any lab, especially for labs handling large volume of samples.
Financial Considerations
One of the key advantages of an automated system will be the better utilization of histotechnologist hands-on time, providing costsavings as economic justifications for an automated IHC system.
16 Apart from the cost of purchasing the equipment, one will also need to consider the other costs related to the purchase (see table below). In addition, the potential cost savings and revenue associated with
the IHC test should also be considered.
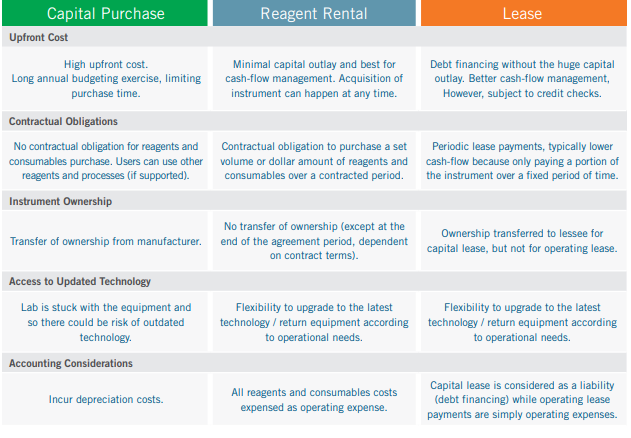
Instrument Acquisition Options
The table below compares three different instrument acquisition options: capital purchase, leasing (either as a capital lease or operating
lease), reagent rental. 17,18,19,20 The financing option could have significant cost implications, and this should be considered in the
context of sample volume (see “Financial analysis” below).
Deciding the Appropriate Acquisition Type
Many labs prefer the reagent rental option as there will be no capital outlay as long as their slide volume is large enough to meet
the reagent purchase requirements. On the other hand, there could be benefits in capital purchase given the long useful life of an
automated IHC slide stainer and the new tax incentives as a result of the updated section 179 deductions as stipulated in the Tax Cuts
and Jobs Acts of 2017. 21,22 A tax professional should be consulted to determine if such tax benefit is applicable to each individual case.
Conduct a Financial Analysis
As discussed, cost savings could be one of the justifications for an IHC automation project.
In order to work out cost savings, the following information will also need to be captured:
Total volume of anatomic pathology tests performed per day
Number of FTE performing IHC tests and hands on time required (most notably hourly rate)
Total volume of IHC tests automated
Total Cost Per Slide (including all reagents and ancillary items)
Revenue generated from each IHC test, if applicable
One of the approaches to estimate the unit cost of an IHC test can be summarized below:
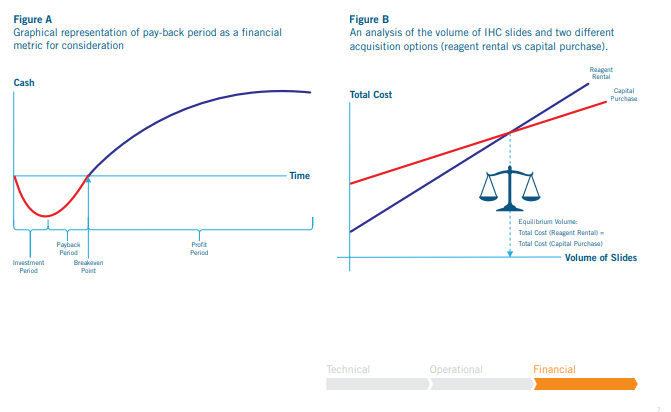
With the financial analysis complete, one can then determine the total cost and revenue based on the volume of samples processed with
automation. With this information, breakeven analysis can be done to determine:
Pay-back period and ROI for the investment (A)
Optimize cost savings and see at what throughput reagent rental agreement is more cost-effective than capital purchase (B)
Increase in revenue as a result decreased FTE/hands-on time and increase samples processed due to automation.
Optimize cost savings/revenue through estimating an appropriate mix of automated and manual IHC tests; or an appropriate mix
of financing options if purchasing more than one instrument is required.
Conclusion
Today, the IHC lab is facing greater challenges to standardize procedures and increase efficiency as pathologists are demanding higher
throughput and quality in the era of personalized medicine. IHC automation is considered one of the key improvements to achieve this
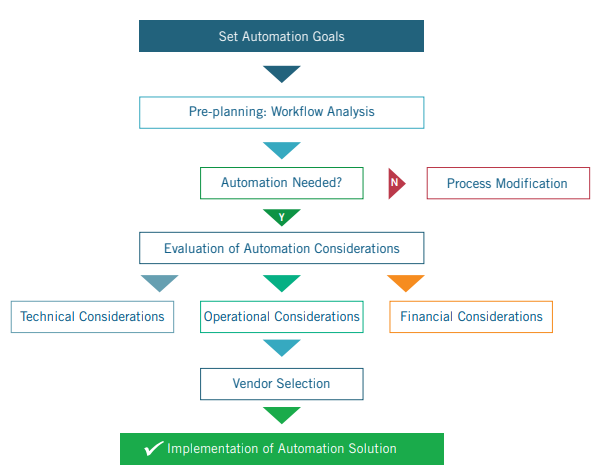
goal. Based on the above discussion, a high-level outline of how to implement IHC automation solution is depicted below. There is no
one perfect standard solution and it is essential for each lab to consider its own constraints and the merits of different options in order
to find an optimized solution to meet their unique needs.
About Biocare Medical
Biocare Medical is a global leader in solutions for cancer research and diagnostics, providing world-class reagents, including tissueconserving simultaneous multiplex antibody cocktails and detections; renowned customer care; and a comprehensive suite of advanced
instrumentation for IHC, molecular, and histology testing. Customers include clinical anatomic pathology labs, pharmaceutical
companies, CROs, and biotechnology companies as well as academic, government, military, and other non-profit labs. Biocare’s reagent
portfolio includes primary antibodies, Multiplex IHC, and FISH probes for target indications. Biocare also offers a unique line of polymer
detections for clinical, human, and animal research that delivers high sensitivity and exceptionally low background. The Company’s
advanced platforms of semi and fully-automated instrumentation have been designed to meet every need from high throughput clinical
diagnostics to flexible research requirements. Biocare Medical’s corporate headquarters and operations are based in the San Francisco
Bay Area with a global distribution network.
References
1. Perkel JM. Immunohistochemistry for the 21 st Century. Science. 2016; 351(6277):1098-1100.
2. Wolcott J, Schwartz A and Goodman C. Laboratory Medicine: A National Status Report prepared for Division of Laboratory Systems National Center for Preparedness, Detection,
and Control of Infectious Diseases Centers for Disease Control and Prevention; 2008.585-606.
3. The American Society for Clinical Laboratory Science. Position Paper: Addressing the Clinical Laboratory Workforce Shortage.;2018
4. Michel RL and Chirstensen S. Dark Daily Special Report: 2008 Trends in Clinical Pathology Laboratory Management;2008
5. Lin F and Chen ZM Standardization of Diagnostic Immunohistochemistry: Literature Review and Geisinger Experience, Arch Pathol Lab Med.; 2014;138:1564–1577
6. Romero LF Sustaining the business of laboratory testing beyond PAMA and reimbursement cuts. Medical Laboratory Observer; 2018; available at https://www.mlo-online.com/
information-technology/lis/article/13009427/sustaining-the-business-of-laboratory-testing-beyond-pama-and-reimbursement-cuts, accessed August 30, 2019.
7. Gonnelli G Planning for laboratory automation. Medical Laboratory Observer; 2017; available at https://www.mlo-online.com/information-technology/automation/
article/13009227/planning-for-laboratory-automation, accessed August 20,2019
8. Lin F and Chen ZM Standardization of Diagnostic Immunohistochemistry: Literature Review and Geisinger Experience. Arch Pathol Lab Med. 2014;138:1564–1577
9.Covill L The LEAN lab: automation, workflow, and efficiency. Medical Laboratory Observer; available at https://www.mlo-online.com/continuing-education/article/13008087/thelean-lab-automation-workflow-and-efficiency. 2015; accessed August 30,2019.
10. Tacker DH, Topardo J, Mahaffey C and Perrotta PL . Workflow Analysis Comparing Manual and Automated Specimen Processing for Mass Spectrometry–Based Vitamin D
Testing. LabMedicine. 2014; 45(4):361-367.
11. Melanson SEF, Linderman NI and Jarolim P. Selecting Automation for the Clinical Chemistry Laboratory. Arch Pathol Lab Med. 2007;131:1063–1069
12. Prichard J, Bitting A and Myers J. Overview of Automated Immunohistochemistry. In: Lin F and Prichard J, ed. Handbook of Practical Immunohistochemistry, New York,
Springer;2011:23-30.
13. Prichard JW. Overview of Automated Immunohistochemistry. Arch Pathol Lab Med. 2014; 138:1578-1582.
14. Volmar KE, Indow MO, Souers RJ, Karcher DS and Nakhleh RE. Turnaround Time for Large or Complex Specimens in Surgical Pathology. Arch Pathol Lab Med.
2015;139:171–177
15. Nakhleh RE What is quality in surgical pathology? J Clin Patho. 2006;59:669–672
16. Ford A. For productivity gains, histology labs press on. CAP Today [serial online]. 2012; available at: http://www.captodayonline.com/Archives/0912/0912b_productivity.html,
accessed September 4,2019.
17. Ridley J. Essentials of Clinical Laboratory Science. New York,Delmar,Gengage Learning; 2010:165-166.
18. Kollmeyer E. Buying Equipment for a Practice Laboratory: Purchase, Lease or Reagent Rental; Manage my Practice Blog; 2011; available at https://managemypractice.com/
laboratory-consultant-libby-knollmeyer-on-buying-equipment-for-a-practice-laboratory-purchase-lease-or-reagent-rental/, accessed August 30, 2019.
19. Hernandez M and Regan K. Blog:To Buy or Not to Buy? Purchasing Decisions in the Clinical Lab; Becker’s Hospital Review; 2014; available at https://www.
beckershospitalreview.com/supply-chain/to-buy-or-not-to-buy-purchasing-decisions-in-the-clinical-lab.html, accessed September 4,2019.
20. Capital Lease vs Operating Lease. Corporate Finance Institute Web site. available at https://corporatefinanceinstitute.com/resources/knowledge/accounting/capital-lease-vsoperating-lease/, accessed August 30,2019.
21. Holland B. How Tax Changes Will Affect New Equipment Strategy & Procurement; Equipment Finance Advisor; 2018; available at http://www.equipmentfa.com/blogs/7559/
how-tax-changes-will-affect-new-equipment-strategy-procurement, accessed September 5,2019.
22. Section 179 Deduction. Official website of Section 179available at https://www.section179.org/section_179_deduction/, accessed September 3,2019.
23. Wiwanitkit V. Purchased Versus Rented Laboratory Instruments, an Example of Situation Analysis on financial Aspects in Thailand, LabMedicine. 2007;38(4):207-212.