A Comparative Study of HIER Methods: Online vs Offline Retrieval
Abstract
The objective of this study is to examine the efficiency and effectiveness of heat induced epitope retrieval (HIER) when performed on a fully automated instrument using single-sided application of a heating element and topical dispense of retrieval buffer solution, commonly referred to as online retrieval, in comparison to offline HIER via the conventional pressure cooker method. Slides from 20 different IHC markers were retrieved both online and offline before undergoing IHC staining using identical staining protocols. Online retrieved slides were retrieved at intervals of approximately 30, 60, and 90 minutes of time to find the minimum threshold to replicate staining quality. The stained slides were then graded through a blind review, and the differences in staining quality scoring between HIER methods were compared. The study found that 75% of graded and scored experimental online HIER slides did not exceed the scores produced by the offline HIER slides under identical staining conditions. Of those 75% of cases, 50% of the online HIER slides scored lower than the offline HIER slide, even at extended retrieval times of up to 90 minutes. Online HIER scored higher than offline HIER only at increased retrieval times of an hour or more. These results demonstrate the limitations of online HIER on fully automated staining instruments when compared to methods of offline HIER performed in a pressure cooker in conjunction with semi-automated staining instruments.
Key Words: Heat induced epitope retrieval (HIER), antigen retrieval, retrieval solution, pressure cooker, online retrieval, offline retrieval,
immunohistochemistry (IHC)
Introduction
In order to examine the morphology of tissues when removed from their body of origin, fixation of the removed tissue must be performed to preserve cellular architecture and prevent the natural processes of putrefaction and autolysis. Formalin, routinely used as a 10% aqueous solution, has long been the standard fixative for tissue processing in the field of histopathology. Reasons for its popularity and long-standing use include its affordability, accessibility, degree of accuracy, relatively high rate of penetration, and its consistency (Thavarajah 2012). As such, formalin fixed, paraffin embedded (FFPE) sections continue to be the gold standard in diagnostic pathology; therefore, most diagnostic criteria has been established for the staining of FFPE tissue. Consequently, the majority of existing histopathology techniques revolve around the mechanisms of formalin fixation (Shi et al. 1997).
It has been found that the formaldehyde present in aqueous formalin exists in the form of methylene glycol, which reacts with tissue proteins to create hydroxymethyl groups (Bogen et al. 2009). These hydroxymethyl groups cross-react with other proteins to form methylene bridges between them, commonly referred to as protein crosslinks (Bogen et al. 2009). This functions to keep tissue components well preserved, however these crosslinks also cause steric interference with antibody binding, preventing immunoreactive staining (Bogen et al. 2009). The usage of formalin as a fixative has then necessitated a procedure to reverse its fixation effects and re-establish immunoreactivity of the desired antigens. This recovery of antigenicity is referred to as antigen retrieval.
Since its inception, antigen retrieval has continued to evolve over the past few decades. Enzyme digestion with proteolytic enzymes was one of the first forms of antigen retrieval developed. Unfortunately, prevention of enzymatic digestion on tissue components is difficult, which causes irreversible loss of tissue morphology, and poses a significant risk of loss of antigenicity (Jasani et al. 2001). In 1991, Shi et al. discovered that heat-mediated methods of antigen retrieval were capable of retrieving a wide range of antigens with decreased risk of morphology loss (Shi et al. 1997). This was groundbreaking in the field of histopathology and gave rise to heat induced epitope retrieval (HIER) as the standard retrieval method. Different methods have been employed for HIER since then, including autoclaving, water baths, steaming, and pressure cooking. However, the most influential variable in all of these methods has appeared to be the temperature to which tissue sections are heated. For example, in studies of optimal retrieval temperature, it has been found that heating to 90°C for 10 minutes produces greater antigenicity than when slides are heated to 60°C for 120 minutes (Shi et al. 1997). Heating in buffers of specific compositions and pH was found to further increase staining intensity, although the exact mechanisms for this are somewhat unclear (Shi et al. 1997). Offline HIER in a pressure cooker remains a popular and reliable method into the present. As increased pressure also increases the boiling points of liquids, the pressure cooker method allows higher temperatures to be reached without causing the solution to boil. Other reasons for its continued prevalence include ease of use for retrieving large batches of slides at a time, higher temperatures decreasing the time required for retrieval, and its relatively cheap equipment costs (Pileri et al. 1999).
Introduction Cont.
Despite innovation and technological advancements, the mechanisms by which HIER methods function still remain poorly understood. It has been hypothesized that high temperature heat treatment of slides disrupts aldehyde cross-links between proteins formed during formalin fixation, which in turn restores antigen epitopes to their native conformations for binding (Krenacs et al. 2010). The temperature to which the tissue is heated, the composition and pH of the retrieval solution used, the volume of solution, and the degree of tissue fixation are all major factors in the effectiveness of HIER (Krenacs et al. 2010). While high temperatures would typically cause denaturation of proteins under normal circumstances, findings show that epitopes for clinical applications require only the primary protein structures to bind, and therefore any potential denaturation of secondary and tertiary protein structure is irrelevant to staining antigenicity (Bogen et al. 2009). Therefore, such high temperatures are essential to effective retrieval, while posing minimal risk to tissue antigenicity.
The diagnostic pathology industry has moved rapidly towards automation over the past two decades as a method of increasing output and decreasing staff requirements in response to ongoing staff shortages and increasing workloads (Prichard, J. W. 2014). To capitalize on this trend, instrument manufacturers have sought to develop instruments capable of executing the entire IHC process, from deparaffinization to counterstaining, so as to require as little staff interaction as possible and thus decrease the need for skilled, experienced laboratory professionals. Such instruments have been referred to as “fully automated” instrument as compared to “semi-automated” instruments which do not perform the deparaffinization and HIER steps. As a result, the methods of HIER performed by fully automated instruments have needed to be redesigned to adhere to the constraints of hardware that must then also proceed with IHC staining on each slide following retrieval. This has resulted in a decrease in platform flexibility and a decrease in user control over selected reagents and protocols.
To incorporate both of these processes into a single instrument design, online HIER is often conducted by applying a heating element directly to the underside of the slide, while dispensing a thin layer of retrieval solution over the top. This way, the same slide module can also be used for the staining protocol, minimizing the instrument’s footprint. Additionally, the incorporation of a slide heating element into the staining instrument offers the advantage of applying heat during slide incubation times, therefore accelerating binding interactions (Prichard, J. W. 2014). However, the speed at which labs have adopted these new retrieval methods over other long-standing, verified methods of retrieval has left a dearth of evidence regarding continuity of quality. There has been little formal comparison testing to examine the differences in staining quality that could result from the online HIER method of one-sided application of heat to the slide and topical dispense of reagent. While the staining quality of these instruments have been approved and validated for clinical use, some studies have found that online HIER fails to successfully produce certain staining results. In a study of MIB-1 immunostaining for diagnosis of hyalinizing trabecular tumors (HTT) of the thyroid, fully automated stainers utilizing online HIER failed to confirm immunoreactivity (Takada et. all, 2018). In this study, four different fully automated staining platforms, including the Leica BOND-MAX, Leica BOND-III, VENTANA BenchMark XT, and Dako Omnis, were used to run HTT positive tissue. Positivity rates of the fully automated platforms ranged from 0-20%, when compared with multiple semi-automated staining methods which produced positivity rates of 30-100% (Takada et. all, 2018). This universal failure of fully automated instruments performing online HIER to match the staining success rates of semi-automated instruments utilizing offline retrieval raises questions about the efficacy of online HIER methods that warrant further exploration.
This study hypothesizes that if HIER is performed online using direct application of a heating element and topically dispensed retrieval solution, then some staining quality will be lost due to decreased efficiency of retrieval. Increased HIER time will be required to achieve equivalent antigen retrieval when compared to the offline HIER method of retrieval in a pressure cooker. This study aims to assess the efficacy of online retrieval by determining how much longer HIER protocols must run to achieve equivalent staining to offline retrieved slides.
Materials and Methods
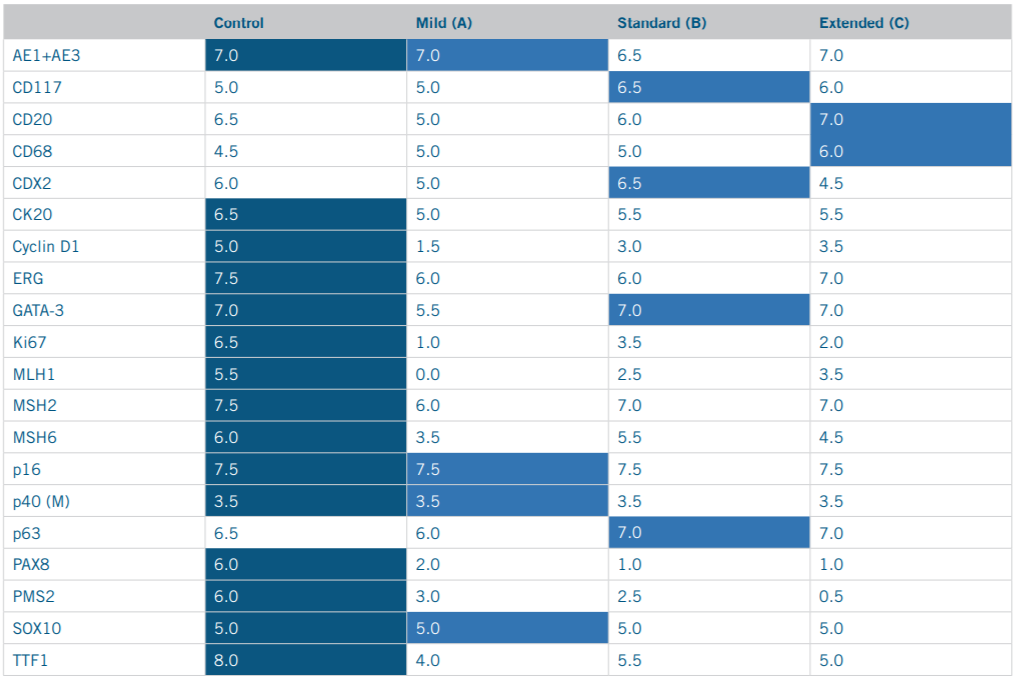
This study was carried out as a quality comparison between offline versus online HIER methods across 20 common IHC markers. The IHC marker selection process took into consideration industry prevalence as well as diversity of disease states and control tissue types in order to compile the list of markers shown in Table 1.
Table 1. Antibodies Selected for Comparison Staining
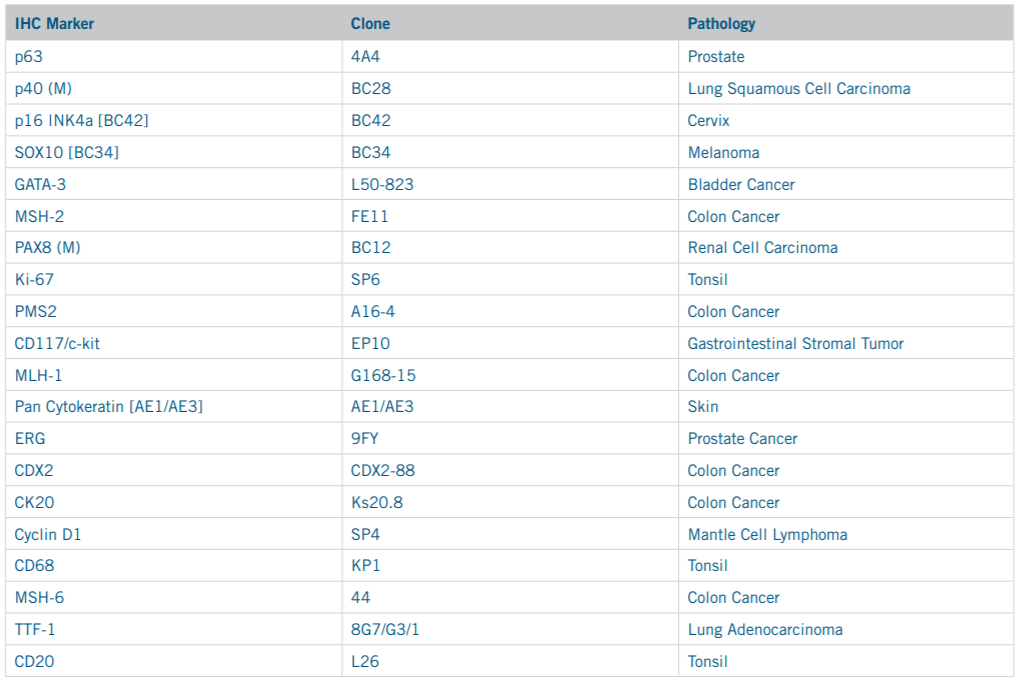
The positivity of each control tissue was verified prior to use and slides were cut in 4μm serial sections to maintain consistent section quality and antigenicity for each slide.
Deparaffinization of slides for staining was performed on a HistoPro ® 3030 H&E Slide Stainer by running slides through three changes of Xylene solution, followed by a series of graded alcohol solutions to water prior to retrieval.
Offline HIER was performed with a Biocare NxGen Decloaking Chamber pressure cooker using Biocare HIER solutions: low pH Diva and Reveal retrieval buffers, and high pH Borg retrieval buffer. This study utilized the standard HIER protocol as recommended by the instrument manufacturer, which is 15 minutes at 110 °C under pressure in the range of 5 – 7 psi, with a total protocol runtime time of approximately 45 minutes.
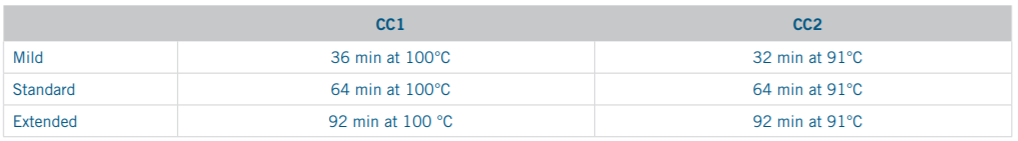
Online retrieval was carried out on the VENTANA BenchMark Ultra instrument. High pH retrieval was performed using the Benchmark ULTRA CC1 solution and low pH retrieval was conducted with application of the Benchmark ULTRA CC2 solution. Slides underwent the Benchmark ULTRA instrument’s pre-programmed deparaffinization protocol in conjunction with the online retrieval. For each of the two retrieval solutions, three standard pre-programmed HIER protocols were used, defined by the manufacturer as “Mild,” “Standard,” and “Extended.”
Table 2. Outline of standard online retrieval protocols
To keep staining conditions as constant and as neutral as possible, all staining was performed without the application of additional heat on an intelliPATH FLX Automated Slide Stainer using prediluted antibodies and reagents developed for room temperature staining. Biocare’s MACH 4 Universal two-step HRP detection system was utilized for IHC detection, and all slides were visualized using a DAB chromogen.
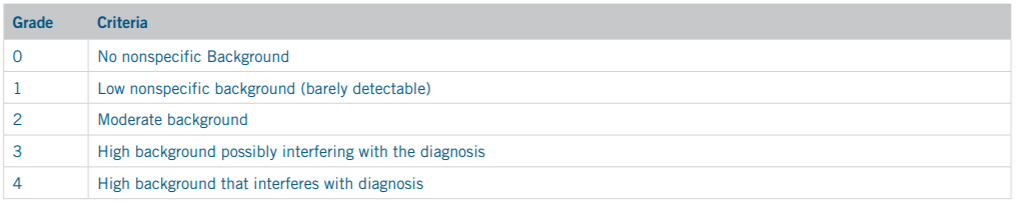
In order to determine the optimal retrieval solution for each marker under both offline and online retrieval conditions, HIER protocols underwent a verification and optimization process. Slides were retrieved with the solution recommended by the antibody manufacturer’s datasheet and optimal staining quality was then verified by a pathologist. In cases where no recommendation for retrieval was available, slides were retrieved with multiple retrieval solutions and their staining quality was assessed to determine which solution produced superior results. All optimization slides were run through the standard IHC staining protocol recommended by the manufacturer for use with the MACH 4 Detection Kit, then sent to an in-house pathologist for blinded confirmation. Slide staining quality was graded according to the standard grading criteria shown in Tables 3 and 4.
Table 3. Staining Positivity Scoring
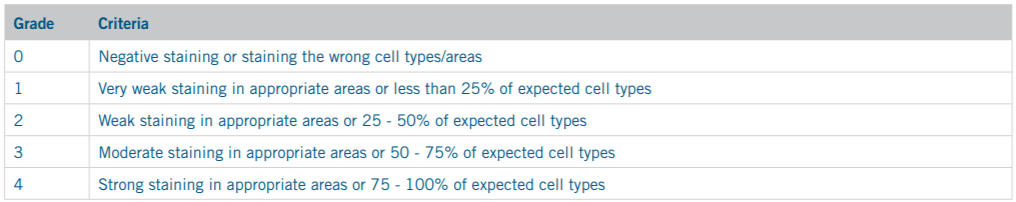
Table 4. Staining Background Scoring
The protocol of the slides that received the best scores in both the offline and the online retrieved groups were selected as the optimal retrieval conditions. This process was repeated for all 20 selected IHC markers.
Once the verification and optimization process was complete, and the optimal retrieval solutions determined, four slides were pulled for each marker, labeled as the Control, A, B, and C.
The Control slides were deparaffinized on the HistoPro ® 3030 H&E Slide Stainer. Following deparaffinization, the slides were then transferred to the Biocare NxGen Decloaking Chamber for offline HIER. Slides A, B, and C, deparaffinized and retrieved on the VENTANA BenchMark ULTRA using the Mild, Standard, and Extended protocols, respectively as described in Table 2.
Afterwards, slides were thoroughly rinsed in DI water, and transferred to a holding TBS buffer solution before being loaded onto the intelliPATH Automated Slide Stainer, where they would be labeled with their corresponding marker. Following staining, slides were oven dried at 50°C, and coverslipped.
The experimental slide sets for each of the 20 markers were then sent to an outside pathology group for review by a group of pathologists and graded by each pathologist for both staining positivity and staining background according to the criteria outlined in Tables 3 and 4.
Since there is an inevitable degree of subjectivity in pathologist slide grading, the positivity and background scores for each HIER protocol were taken as an aggregate of all pathologists’ scores to account for any variation. Next, a total score was generated, referred to in this study as a “quality” score, by subtracting the aggregate background value from the aggregate positivity value. By this method, higher quality scores imply greater positivity with lesser background, with a maximum score of 8 and a minimum score of -8.
Quality Score = [Aggregate Positivity Score] – [Aggregate Background Score]
Results
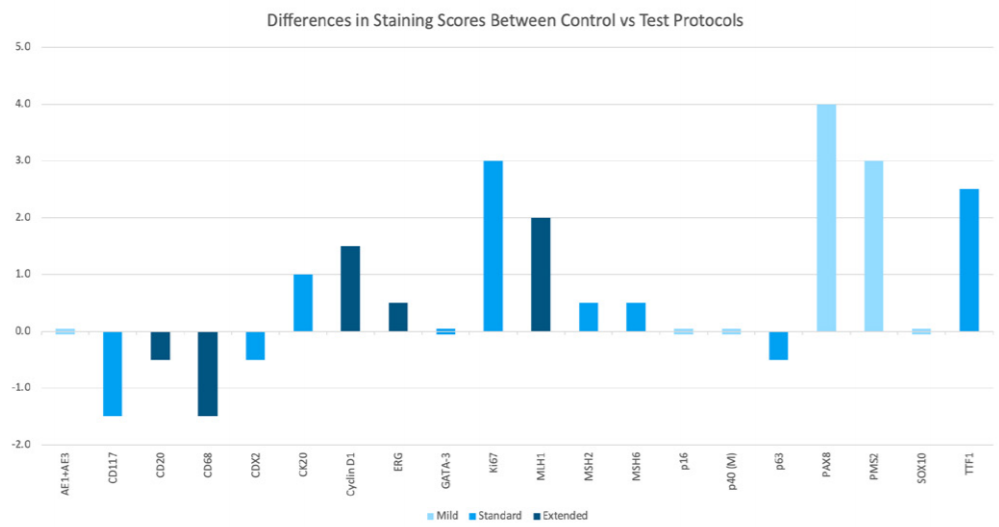
For each of the 20 IHC Marker slide sets (Control, A, B, and C), the total quality scores of each slide were recorded and compared, and the numeral differences between the control slide and each online HIER test slide were noted.
In 10 of the 20 slide sets, all online HIER test slides scored lower than the offline HIER control. In 5 of the slide sets, an online HIER slide received an equivalent score to the control slide, and in another 5 of the slide sets, an online HIER slide scored higher than the control slide. The retrieval protocol time was analyzed for online HIER slides that scored equivalent or higher than the control, to determine the minimum amount of retrieval time that was required to achieve equal or higher scoring.
The results showed that of the 5 slide sets where an online HIER slide showed equivalent staining (AE1/AE3, GATA-3, p16, p40, and SOX10), 4 achieved equivalent staining under the nearly 30 minute Mild online HIER protocol, and 1 required the approximately 60 minute Standard online HIER protocol.
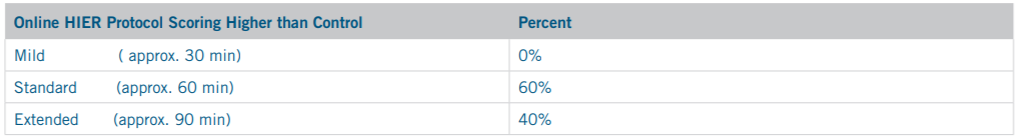
Of the 5 slide sets where an online HIER slide scored higher than the offline HIER Control (CD117, CD20, CD68, CDX2, and p63), 3 had been retrieved using the 60 minute Standard online HIER protocol, and 2 had required the approximately 90 minute Extended online HIER protocol. No higher scoring online HIER slides had been retrieved using the Mild HIER protocol.
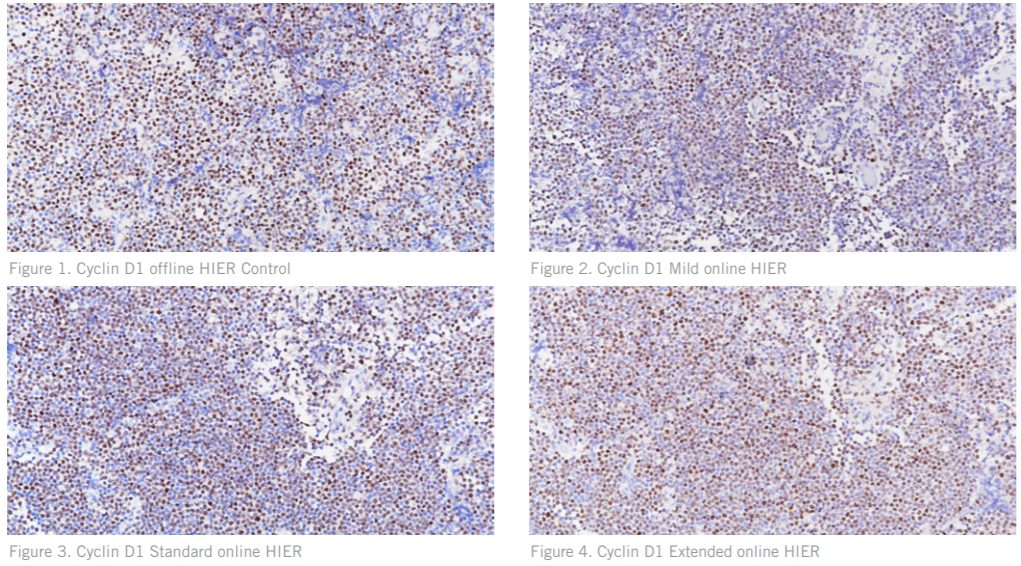
Figures. 1 – 4 Cyclin D1 stained on tissue positive for Mantle Cell Lymphoma showing reduced positive staining intensity and higher background on the online HIER test slides as compared to the control
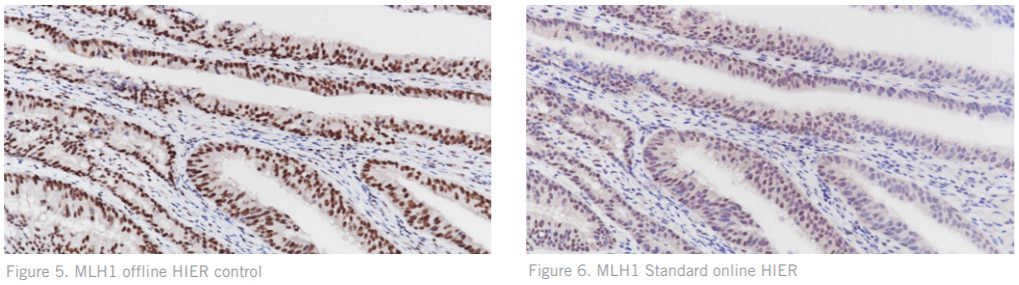
Figures. 5 – 6 stained for MLH1 on cancerous colon tissue illustrating the difference in staining quality between offline and online HIER
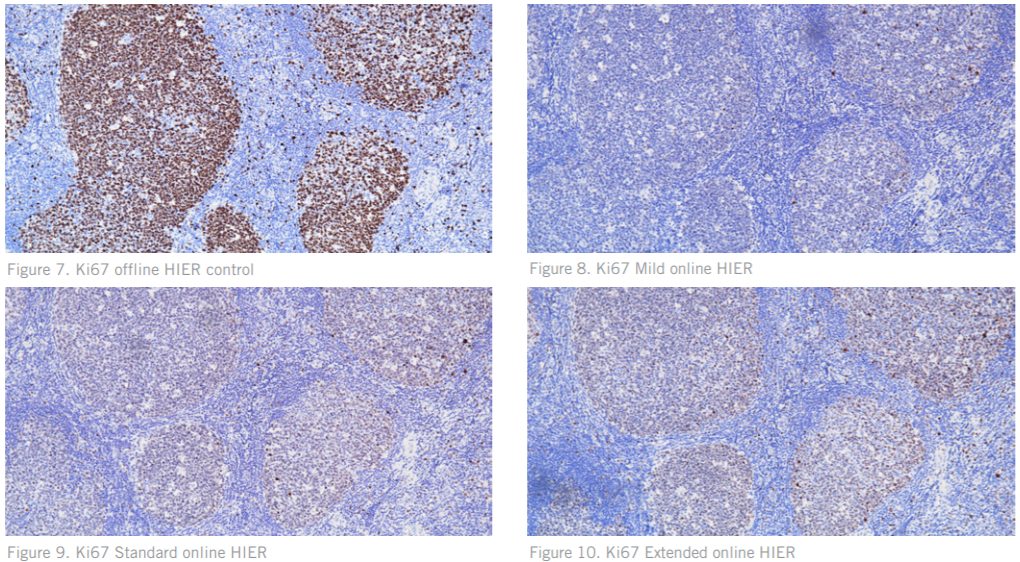
Figures. 7 – 10 Ki67 on normal tonsil showing a dramatic difference in staining quality between the offline HIER slide and online HIER slides. Figures 8-10 show decreased positive signal, with the Standard HIER (Figure 9) showing increased background.
These results are displayed in Table 5. For each marker, the highest slide score in the slide set is highlighted. In sets showing equal scoring between the control and an online HIER test slide, both the control and equivalent test slide are highlighted. In sets where multiple online HIER test slides had equal scoring, the shorter incubation time was prioritized and highlighted.
Table 5. Quality scores of offline control slides and online test slides were given for each. In each slide set, the highest scoring slides are highlighted. Equal scoring Control and Test slides are both highlighted.
Table 5. Quality Scores by Marker and Retrieval Protocol
In total, the offline HIER protocol performed equivalent or better than all online HIER protocols in 15 out of 20 or 75% of cases Table 6.
Table 6
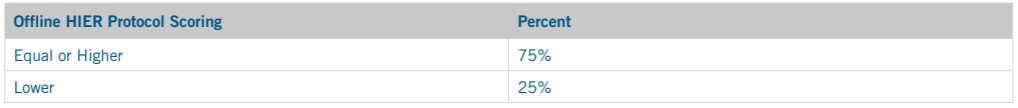
The differences in value between the Control and highest scoring test slides in each set were calculated and visualized in Figure 11 for further analysis. As displayed in the graph, the average difference in scoring between the Control slide and highest scoring test slide was larger in sets where the Control slide scored highest than in sets where an online HIER test slide scored highest.
On average, the offline HIER control slides performed better than the highest scoring online HIER test slide by a margin of 1.65 points, whereas the online test slides that scored higher than the offline control did so by an average margin of 0.9 points.
In other words, in cases where the offline HIER control slide performed better than the online HIER test slides, it did so by a higher margin.
Figure 11 Differences in staining scores between control slide vs highest scoring test slides visualized
In the 5 cases where an online HER protocol slide scored higher than the offline HIER control slide score, 3 required the Standard HIER of approximately 60 minutes to do so, while 2 required HIER of up to 90 minutes in the Extended HIER protocol to match the control slide score. No slides retrieved for under 60 minutes were able to match the control slide score. These results are shown in Table 7.
Table 7
Discussion
The findings of Takada et al. in their study, Re-evaluation of MIB-1 immunostaining for diagnosing hyalinizing trabecular tumour of the thyroid, indicated discrepancies in the robustness of staining between fully automated and semi-automated instruments, but did not go so far as to identify the source of this discrepancy. The data of this study regarding differences in staining quality resulting from online vs offline HIER appears to confirm that online HIER utilizing single-sided application of a heating element coupled with topical application of a retrieval solution is not as robust a method of HIER for IHC when compared to offline HIER performed in a pressure cooker, and is a probable cause of staining discrepancies. This study’s findings show that offline HIER performed equal to or better than online HIER in 75% of cases. In 50% of cases, online HIER failed to match the quality of staining produced by online HIER completely, even when online HIER times were extended to 92 minutes. In the 25% of cases where online HIER slides scored higher than the offline HIER control, this was only able to be achieved when HIER times were extended to an hour or more.
If the antigen retrieval method is not as robust, steps must be taken to compensate for this in order to achieve comparable staining quality. For example, incubation steps may need to be increased to give antibodies more time to bind their antigens. However, this would be impractical in the context of a clinical diagnostic laboratory. If incubation time is not to be increased, the most common methods of increasing staining quality are to increase the temperature of the staining environment, as well as to increase the protein concentrations of reagents. Implementation of either of these methods would result in an increased rate of antibody-antigen binding and therefore increase staining quality.
When IHC staining is performed on a fully automated instrument, heat is typically applied through all staining steps, which would serve to increase antigen binding through an increase in staining temperature. By this method, the applied heat would increase molecular motion in solution, accelerating the rate of antibody-antigen binding within a given amount of time. This design feature may point to compensation for a less effective HIER process, which must be supplemented through increased antibody-antigen binding. An additional method to enhance staining quality would be to increase the sensitivity of the detection system by increasing the protein concentration of reagents. Through this method, a greater concentration of antibodies present in solution would facilitate increased binding activity. However, it must be noted that both enhancement methods would also increase the required resources for staining protocols, thus both increasing cost.
It should also be noted that while an HIER protocol may maintain retrieval temperature for a given amount of time during the HIER step, the total time for the HIER procedure extends beyond that. Online HIER performed with a direct heating element and offline HIER performed in a pressure cooker both require a period of “ramping up” to the required temperature and/or pressure for HIER, maintaining the required temperature and/ or pressure for an established amount of time, and then undergoing a “cooldown” process back to ambient temperature and/or pressure. These ramp up and cool down steps both take up a significant amount of the total time.
The entire procedure for offline HIER accounting for deparaffinization, loading, pressure cooking, cooldown, and transfer, took no more than 90 minutes (1.5 hours). Whereas, the VENTANA BenchMARK ULTRA’s Online deparaffinization and HIER amounted to a total runtime of approximately 120 minutes for the Mild HIER protocol (2 hours), 150 minutes (2.5 hours) for the Standard HIER protocol, and 180 minutes (3 hours) for the Extended HIER protocol.
In short, the total procedure for online HIER performed by the application of a direct heating element appears to take considerably more time than offline HIER in a pressure cooker. These longer procedure times could potentially have significant implications for a lab’s workflow and output. Given this, it is logical to assume that this would be another reason that a fully automated instrument with these HIER times would need to increase antibody-antigen binding rates in order to supplement the time taken by the retrieval process. This aspect would necessitate heat application through all staining steps, and/or an increase in reagent protein concentration, in order to shorten incubation times and create protocol durations comparable to those of semi-automated instruments employing more robust HIER methods.
Limitations
The HIER solutions could not be kept constant between the two HIER methods due to the closed nature of the VENTANA BenchMark ULTRA instrument. The instrument software does not allow for any substitute HIER solutions and its mechanism of action, whereby solutions applied to the slide are further diluted while running, is prohibitive. Additionally, 36 minutes for the Mild HIER protocol was used as the minimum online HIER time, compared to 15 minutes for the offline HIER time, since each were recommended as standards according to the manufacturer. This study did not run an equivalent 15 minute Online HIER protocol, assuming that the longer recommended time accounted for lower HIER temperature and lack of pressure found in the Online HIER protocols, and would therefore would be the minimum time still likely to be successful. It is possible that some markers would have achieved equivalent staining with a lesser HIER time, though the time and software constraints of this study made additional tests of shorter HIER times impractical.
Deparaffinization between the two HIER methods was conducted using two different methods. Deparaffinization for the online HIER slides was performed on the VENTANA BenchMark ULTRA instrument, to account for both procedures typically being performed online together as part of the instrument’s fully automated staining protocols.
Due to time and resource constraints, experimental staining was conducted on 20 markers on a single fully automated instrument. It is unknown if the same limitations in online HIER would also be seen on comparable fully automated instruments such as the Leica BOND III, or on additional IHC markers. It would be beneficial to extend this study in the future to include more markers and other online HIER platforms.
Conclusion
This study asserts that the method of online HIER performed through the single-sided application of a heating pad with topical application of retrieval solution appears to be less effective and less efficient when compared to offline HIER performed by submersion in retrieval solution in a pressure cooker. The online HIER method would appear to require implementation of techniques to increase the rate of antigen binding, including increasing temperature and increasing detection sensitivity, in order to offset the loss of antigenicity due to weaker HIER. These modifications would hypothetically result in an increase of resources and therefore an increase in cost. Focuses of future studies would include repeating this study model with different IHC markers of interest and on a different platform for online HIER to confirm results. The literature would also benefit from repeating this procedure in the inverse, determining what effects offline HIER in a pressure cooker has when stained on a fully automated instrument with heat. Additionally, further comparison studies may corroborate these findings by measuring the difference in reagent protein concentration required to achieve equivalent staining from online HIER slides compared to offline HIER slides retrieved in a pressure cooker.
Declaration of Interests
The author declares the following financial interests and personal relationships which may be considered as potential competing interests: EmilyItoku is an employee of Biocare Medical LLC. Funding for this study was provided by Biocare Medical LLC.
Dako, Autostainer and Omnis are registered trademarks of Agilent Technologies. Leica, BOND-MAX and BOND-III are registered trademarks of Leica. Roche, Ventana, Benchmark XT and Benchmark ULTRA are registered trademarks of Roche.
Literature Cited
1. Thavarajah, R., Mudimbaimannar, V., Rao, U., Ranganathan, K., & Elizabeth, J. (2012). Chemical and physical basics of routine formaldehyde fixation. Journal of Oral and Maxillofacial Pathology, 16(3), 400. doi:10.4103/0973-029x.102496
2. Shi, S., Cote, R. J., & Taylor, C. R. (1997). Antigen Retrieval Immunohistochemistry: Past, Present, and Future. Journal of Histochemistry & Cytochemistry, 45(3), 327-343. doi:10.1177/002215549704500301
3. Bogen, S., Vani, K., & Sompuram, S. (2009). Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced cross-links. Biotechnic & Histochemistry, 84(5), 207–215. doi:10.3109/10520290903039078
4. Jasani, B., & Rhodes, A. (2001). The role and mechanism of high-temperature antigen retrieval in diagnostic pathology. Current Diagnostic Pathology, 7(3), 153–160. doi:10.1054/ cdip.2001.0076
5. Pileri, S. A., Roncador, G., Ceccarelli, C., Piccioli, M., Briskomatis, A., Sabattini, E., . . . Falini, B. (1999). Antigen retrieval techniques in immunohistochemistry: Comparison of different methods. Journal of Pathology, 183(1), 116-123. doi:10.1002/(SICI)1096-9896(199709)183:13.0.CO;2-2
6. Krenacs L., Krenacs T., Stelkovics E., Raffeld M. (2010) Heat-Induced Antigen Retrieval for Immunohistochemical Reactions in Routinely Processed Paraffin Sections. In: Oliver C., Jamur M. (eds) Immunocytochemical Methods and Protocols. Methods in Molecular Biology (Methods and Protocols), vol 588. Humana Press. doi:10.1007/978-1-59745-324-0_14
7. Prichard, J. W. (2014). Overview of Automated Immunohistochemistry. Archives of Pathology & Laboratory Medicine, 138(12), 1578-1582. doi:10.5858/arpa.2014-0083-ra
8. Takada, N., Hirokawa, M., Ohbayashi, C., Nishikawa, T., Itoh, T., Imagawa, N., . . . Miyauchi, A. (2018). Re-evaluation of MIB-1 immunostaining for diagnosing hyalinizing trabecular tumour of the thyroid: Semi-automated techniques with manual antigen retrieval are more accurate than fully automated techniques. Endocrine Journal, 65(2), 239-244. doi:10.1507/endocrj.ej17-0413

