2021 CMS Payment Modifications in Pathology
Since 1992, Medicare has paid for the services of physicians and other billing professionals under the physician fee schedule (PFS).
Since 1992, Medicare has paid for the services of physicians and other billing professionals under the physician fee schedule (PFS). Physicians’ services paid under the PFS are furnished in a variety of settings, including physician offices, hospitals, ambulatory surgical centers, and clinical laboratories. For many diagnostic tests and a limited number of other services under the PFS, separate payment can be made for the professional and technical components of services.
The technical component is frequently billed by suppliers like independent diagnostic testing facilities, while the professional component is billed by the physician or practitioner.¹
In 2019, the Centers for Medicare & Medicaid Services (CMS) finalized broad changes related to evaluation and management (E/M) services to reduce administrative burden, improve payment rates, and reflect current clinical practice. The health care community supported restructuring and revaluing the office-based E/M codes, which will increase payments for primary care and other officebased services. Unfortunately, by law, any changes to the PFS cannot increase or decrease expenditures by more than $20 million. To comply with this budget neutrality requirement, any increases must, therefore, be offset by corresponding decreases. CMS estimates that the 2021 policies will increase Medicare spending by $10.2 billion, necessitating steep cuts by reducing the Medicare conversion factor.2
On December 1, 2020, (CMS) issued a final rule that included updates on policy changes for Medicare payments under the PFS, and other Medicare Part B issues, that were scheduled to take effect on January 1, 2021. Those updates would have resulted in a 9% overall reduction in Medicare payments for the pathology field.1,2 Fortunately, the Consolidated Appropriations Act, 2021 was signed into law on December 27th, 2020 which brought COVID-19 relief funding and mitigated the proposed decreased reimbursement for 2021 with $3 billion in additional funding.
Under the initial 2021 PFS final rule, CMS issued a conversion factor of $32.41.
Upon passage of the act, CMS’ updated conversion factor is now $34.89.4
Despite this recent bit of positive news, laboratories and pathology practices continually seek options to reduce their operational costs and improve efficiencies. For those laboratories performing routine immunohistochemistry (IHC) staining, a minimally intrusive method to meet this goal may be achieved through the consideration of alternative vendors for their primary antibody needs. In 2014, the College of American Pathologists (CAP) updated its standards within the Anatomic Pathology Checklist and recommended that laboratories changing vendors of a primary antibody, provided that said antibody is the same clone, would only need perform a confirmation of an assay’s performance with at least 2 known positive and 2 known negative cases.3 In addition, laboratories may also consider transferring a portion of their single-antigen tests commonly performed in a panel to a multiplex (or multi-antigen) cocktail. This would allow for preservation of patient sample tissue, cost savings via reduction of the overall slide workload, and more efficient interpretation. When considering the technical payment for IHC procedures, the transition of two single-antigen tests to multiplex may allow for an overall increase in reimbursement. There are select IHC vendors that offer primary antibodies and multiplex cocktails specifically formulated for use on a variety of IHC instrumentation and/or manual applications which could be considered.3
To learn more about improving your labs efficiency, automation and cost saving options available for your laboratory, please contact Biocare
Medical at 1-800-799-9499 or visit our website at www.biocare.net
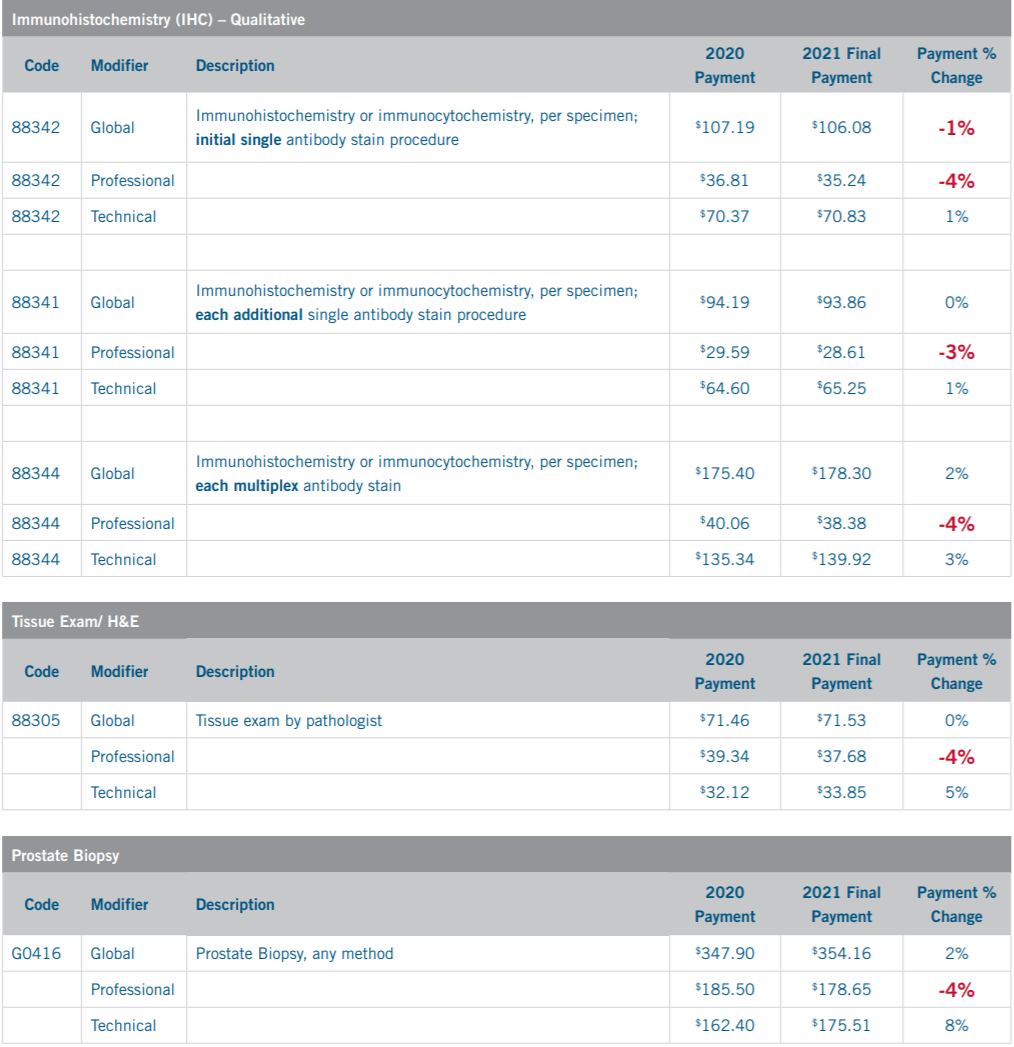
CMS Physician Fee Schedule 2020 vs 2021 Payment Change – National Average
1. Centers for Medicare & Medicaid Services. (2020, December 01). Fact Sheet – Final Policy, Payment, and Quality Provisions Changes to the Medicare Physician Fee Schedule for Calendar Year. CMS.gov. https://www.cms.gov/ newsroom/fact-sheets/final-policy-payment-and-quality-provisions-changes-medicare-physician-fee-schedule-calendar-year-1.
2. College of American Pathologists. (2020, September 10). Medicare Payment Cuts Factsheet. CAP.org. https://documents.cap.org/documents/medicare-payment-cuts-fact-sheet.pdf.
3. Anatomic Pathology Checklist, 2014. Laboratory Accreditation Program. College of American Pathologists. Waukegan, IL.
4. Boese, J. (2021, February). Health Care Policies in the Consolidated Appropriations Act, 2021. https://www.claconnect.com/resources/articles/2021/health-care-policies-in-the-consolidated-appropriations-act-2021

