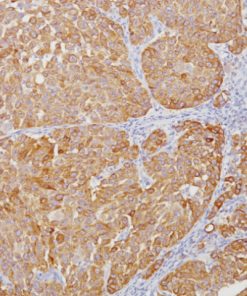
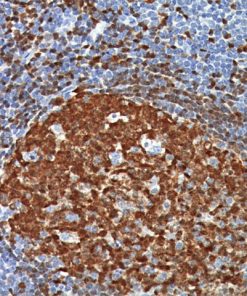
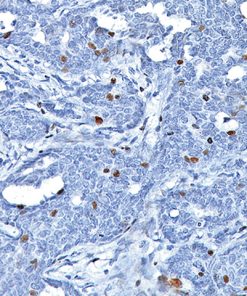
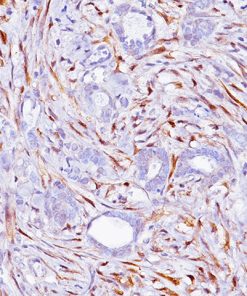
Lambda Light Chain [N10/2]
$150.00 – $386.00
Description
Product Description
This antibody recognizes lambda light chains of human immunoglobulins, which may be useful in the identification of leukemias, plasmacytomas, and certain non-Hodgkin’s lymphomas (1-5). The most common feature of these malignancies is the restricted expression of a single light chain class. The normal human kappa/lambda ratio is approximately 2:1. The presence of clear cut light chain restriction with a kappa/lambda ratio more than 10:1 is consistent with a malignant proliferation (2,6).
Specifications
Specifications
| Intended Use | |
|---|---|
| Format | |
| Volume | |
| Source | |
| Clone | |
| Isotype | |
| Antigen | |
| Localization | |
| Positive Control |
Datasheets & SDS
References
1. Samoszuk MK, et al. Limitations of numerical ratios for defining monoclonality of immunoglobulin light chains in B-cell lymphomas. Diagn Immunol. 1985; 3(3):133-8.
2. Bray M, Alper MG. Lambda light chain predominance as a sign of emerging lymphoma. Am J Clin Pathol. 1983 Oct; 80(4):526-8.
3. Sobol RE, et al. Use of immunoglobulin light chain analysis to detect bone marrow involvement in B-cell neoplasms. Clin Immunol Immunopathol. 1982 Jul; 24(1):139 -44.
4. Falini B, et al. Double labeled-antigen method for demonstration of intracellular antigens in paraffin-embedded tissues. J Histochem Cytochem. 1982 Jan; 30(1):21-6.
5. Marshall-Taylor CE, et al. Immunohistochemical detection of immunoglobulin light chain expression in B-cell non-Hodgkin lymphomas using formalin-fixed, paraffinembedded tissues and a heat-induced epitope retrieval technique. Appl Immunohistochem Mol Morphol. 2002 Sep; 10(3):258-62.
6. Kremer M, et al. Immunohistochemistry in bone marrow pathology: a useful adjunct for morphologic diagnosis. Virchows Arch. 2005 Dec; 447(6):920-37.
7. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
8. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline-Fourth Edition CLSI document M29-A4 Wayne, PA 2014.
Related products
Primary Antibodies
B
B


![Tonsil stained with Lambda Light Chain [N10/2] antibody.](https://biocare.net/wp-content/uploads/3063.jpg)